Osmometry & Isotonic Drink Analysis
Isotonic is a relative term that describes the solute concentration of a solution as like that of human blood. It is a common nutritional claim familiar to many consumers and is routinely used as a marketing tool for numerous different beverage types (beer, energy drinks, sports drinks, etc.). However, there is a distinct lack of clarity surrounding the health benefits and veracity of many isotonic drinks available to consumers. Although this is a common occurrence when any scientific concept enters general vernacular, this uncertainty may extend into the production line.
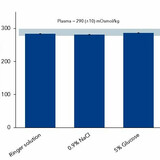
Osmometry is the foremost tool available for assessing the isotonicity of a solution. It is used to substantiate the osmolality of beverages according to the relevant regulations. For example, the European Food Safety Authority (EFSA) states that isotonic drinks must have an osmolality of 270 – 330 mOsmol/kg. This is the expression of a solution’s concentration as the total number of solute particles by weight of solvent. It is a complex measurement that is extremely difficult to quantify without osmometry.
In this blog post, Knauer outlines how to check the isotonicity of your beverages.