
Lipid nanoparticles (LNPs) became famous for the rapid development and largescale production of vaccines during the COVID-19 pandemic. Theses vaccines consist of mRNA coding for an antigen encapsulated in LNPs. Because the mRNA is sensitive to inactivation by distinctive oxidation products of the lipids, special care in quality control of the formulations and raw materials is required. Here, we suggest a workflow for HPLC-MS/MS detection of lipid related impurities in LNP formulations, independent of impurity standards.
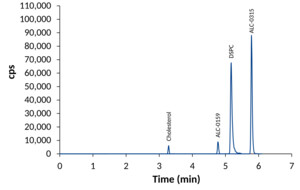
Overlay of XIC of quantifier transitions for lipids of the vaccine BNT162b2.

Workflow for the selection of transitions for lipid impurities. A: Expected impurity, known from literature review [3,4]. B: Chromatogram of the parent lipid ALC-0315, detected in ESI+, Q1MI mode with Q1 mass = 782.7. Three peaks were found for the monito
| Methode | LC-MS |
|---|
| Flussrate | 0.4 ml/min |
|---|---|
| Modus | RP |
| Injektionsvolumen | 2µL |
| Substanzen | Cholesterol, DSPC, ALC-0315, ALC-0159, ALC-0315 N-oxide |
| Schlüsselwörter | LC-MS, LNP, lipids, impurities, purity, N-oxide |
| CAS Nummer | 57-88-5, 816-94-4, 2036272-55-4, 1849616-42-7 |

Wir helfen Ihnen gerne!