Science with Passion
Application No.: VPH0081 Version 1 07/2024
CBD purification –
Part 2: Transfer and optimization of a purification method in preparative scale
J. Wesolowski, Y. Krauke, G. Greco; krauke@knauer.net
KNAUER Wissenschaftliche Geräte GmbH, Hegauer Weg 38, 14163 Berlin
Photo/Collage: KNAUER
Summary
The purification of natural products can be achieved by various techniques, including preparative single- and multi-column liquid chromatography. However, the development of efficient, fast and cost-effective methods for the purification of natural products from real samples remains a difficult and challenging task due to the complexity of the matrix. In contrast to conventional purification methods such as distillation, preparative chromatography uses less energy, consumes less water and chemicals while achieving higher yields [1].
In this application, a batch process for the purification of CBD (cannabidiol) from a CBD-rich cannabis extract was developed and optimized. Using the batch process, approximately 137 mg of CBD with a recovery of 95 % and a purity of 97 % was obtained from a 4 ml extract.
Introduction
Single column batch processes are generally used as a chromatographic purification technique. In this technique, a defined volume of the sample is injected with a suitable solvent, into the system. The sample is then transported through the column by a continuous flow of the solvent and ideally the single components separated from each other due to different interactions with the column. The eluate is analysed at the outlet of the column using a suitable detector and the target substance collected by fractionation. This can be done either by a fraction collector or by a valve. Ideally, the fractions then contain the desired product with the required purity.
System Setup
The purification was performed with a KNAUER AZURA® preparative HPLC system. For the preparative HPLC system, an assistant module, a fraction collector, and a preparative HPLC pump with 250 ml/min and ternary LPG module were used. The assistant was configured with a detector, an 8-port 4-positions injection valve and sample pump with a 10 ml pump head. The injection valve was used with different sample loops which were loaded via the sample pump or manually using a syringe. The detection was carried out with a single-channel wavelength detector (190 to 500 nm) and a semi-preparative flow cell (Fig. 1)

Fig. 1 System setup of preparative HPLC system; left: fraction collector; middle: dynamic mixer; right from top to bottom: eluent tray with bottles, assistant module with column holder and column, pump.
Due to the progressive decriminalization of cannabis for recreational and medicinal use, the properties of this controversial plant have gained increasing attention. To date, more than 500 phytochemicals have been identified in Cannabis sativa L. [2]. These include the medically important cannabinoids, but also terpenes, flavonoids and other components [3]. Cannabinoids are a group of terpenophenol compounds with a characteristic C21 framework (or C22 if they are present in acidic form), which are responsible for many therapeutic effects [4]. One of the most known cannabinoids, besides THC (tetrahydrocannabinol), is CBD. CBD has attracted a lot of attention in recent years due to its potential health benefits [5]. It is often used to relieve pain, inflammation and anxiety [5]. However, the purification of CBD and other cannabinoids from cannabis extracts presents a challenging task[6]. This is mainly due to the complexity of the matrix, which contains terpenes, waxes, chlorophylls and other cannabinoids [7]. In addition, the chemical structures and physical properties of cannabinoids are similar, making them difficult to separate. Therefore, specific methods and techniques are required to ensure accurate separation. Batch processes are generally used as a chromatographic purification technique. Here, we show the purification of CBD from a CBD-rich cannabis extract.
Sample Preparation
Before injection, the ethanol based cannabis extract was filtered with a PTFE filter (0.20 µm).
Results
Method optimization and overload studies
Using the obtained method from the scale up experiments (VPH0080), subsequent measurements were performed with the extract sample on the preparative system to purify CBD. For easier handling and better reproducibility premixed solvents were used; 70:30 ethanol/water for the separation and 90:10 ethanol/water for cleaning of the column. The CBD-rich extract was then injected three times. Fig. 2 shows the overlay of the chromatograms highlighting the reproducibility of the method.
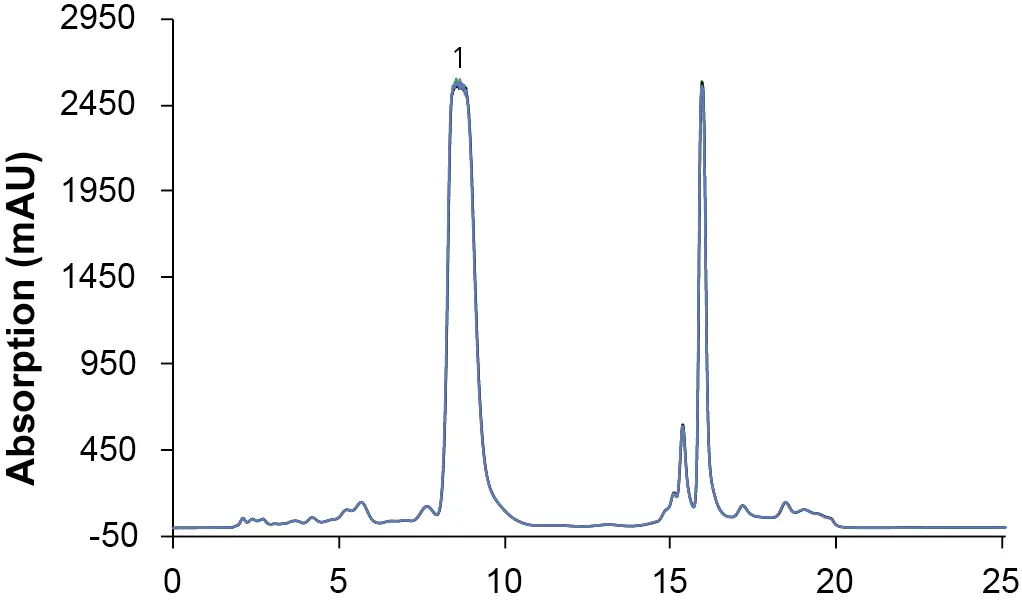
Fig. 2 Overlay chromatograms of three preparative measurements with premixed eluents; sample: CBD-rich extract; 1: CBD; ES II 100-15 C18 250 x 20 mm; 25 ml/min; 200 µl injection.
After establishing the preparative method, volume overload studies were carried out to determine the maximum loading capacity of the preparative column. Fig. 3 shows the chromatograms of the overloading studies on the preparative column with increasing injection volumes of the CBD-rich cannabis extract in an overlay plot. The focus was on the absorption behaviour of the target CBD. A clear peak broadening and increasing fronting was observed with increasing injection volumes. With an injected volume of 5 000 µl, no clear separation of CBD and the earlier eluting peaks was observed.
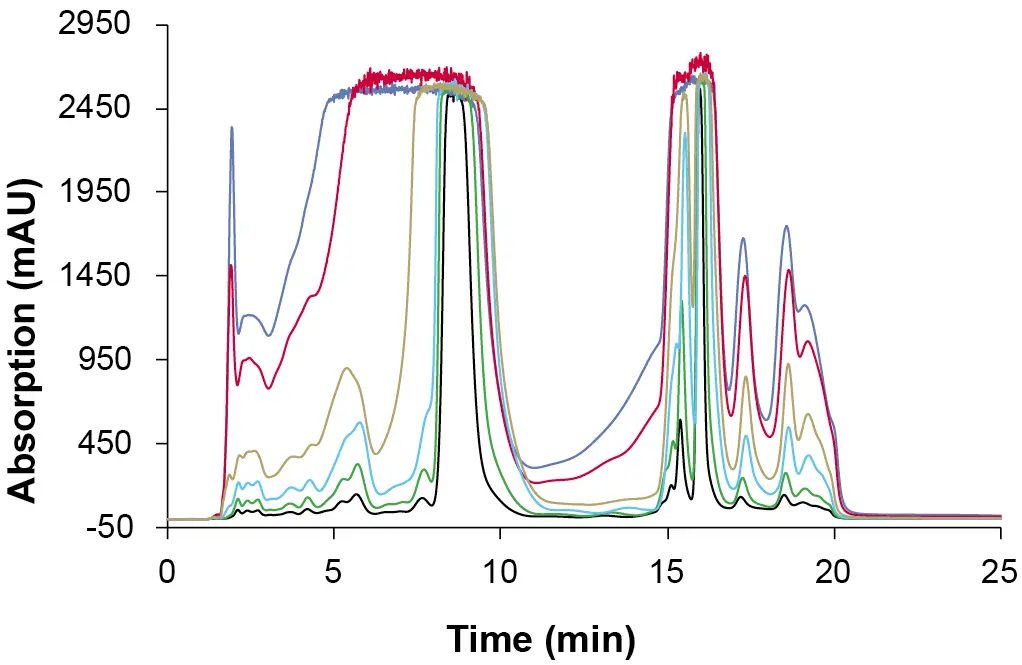
Fig. 3 Overlay chromatogram of preparative overload tests. Sample: CBD-rich extract; black: 200 µl; green: 500 µl, blue: 1 000 µl; yellow: 2 000 µl; red: 4 000 µl; purple: 5 000 µl; ES II 100-15 C18 250x20 mm; 25 ml/min.
Fractionation
In order to achieve high throughputs, an injection volume of 4 000 µl was chosen. Furthermore, to save solvent and time, the run time was reduced from 25 to 23 minutes. The fractionation window was defined over the peak range of CBD between minute 3 and 12. The automatic fractionation was carried out with a fraction volume of 12.5 ml. The chromatogram in Fig. 4 shows the result with the 18 collected fractions. The collected fractions and the extract sample were analyzed using the previously described acetonitrile method [8].
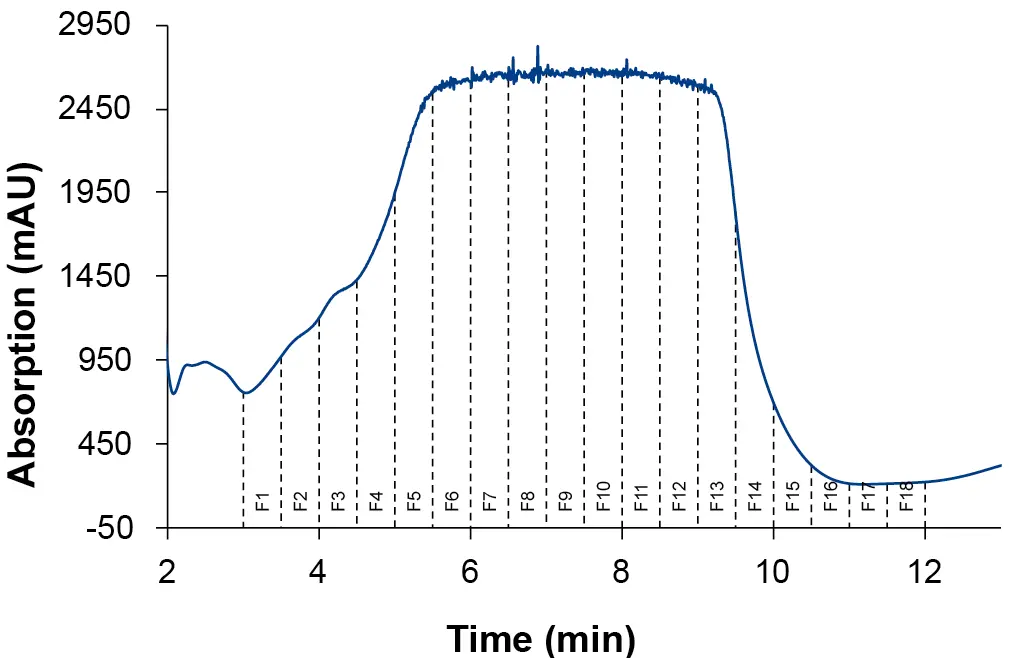
Fig. 4 Chromatogram section of preparative fractionation over CBD peak. 12.5 ml fractions (F1 to F18); ES II 100-15 C18 250 x 20 mm; 4 000 µl injection.
The results from the fraction analysis are compared with the analysis results of the injected CBD-rich extract sample in the following Tab. 1. In the fractions where CBD could be quantified, the amount, purity, and recovery of CBD in each fraction were determined.
Tab. 1 Amount, purity and recovery of CBD in fractions with an injection volume of 4 000 µl.
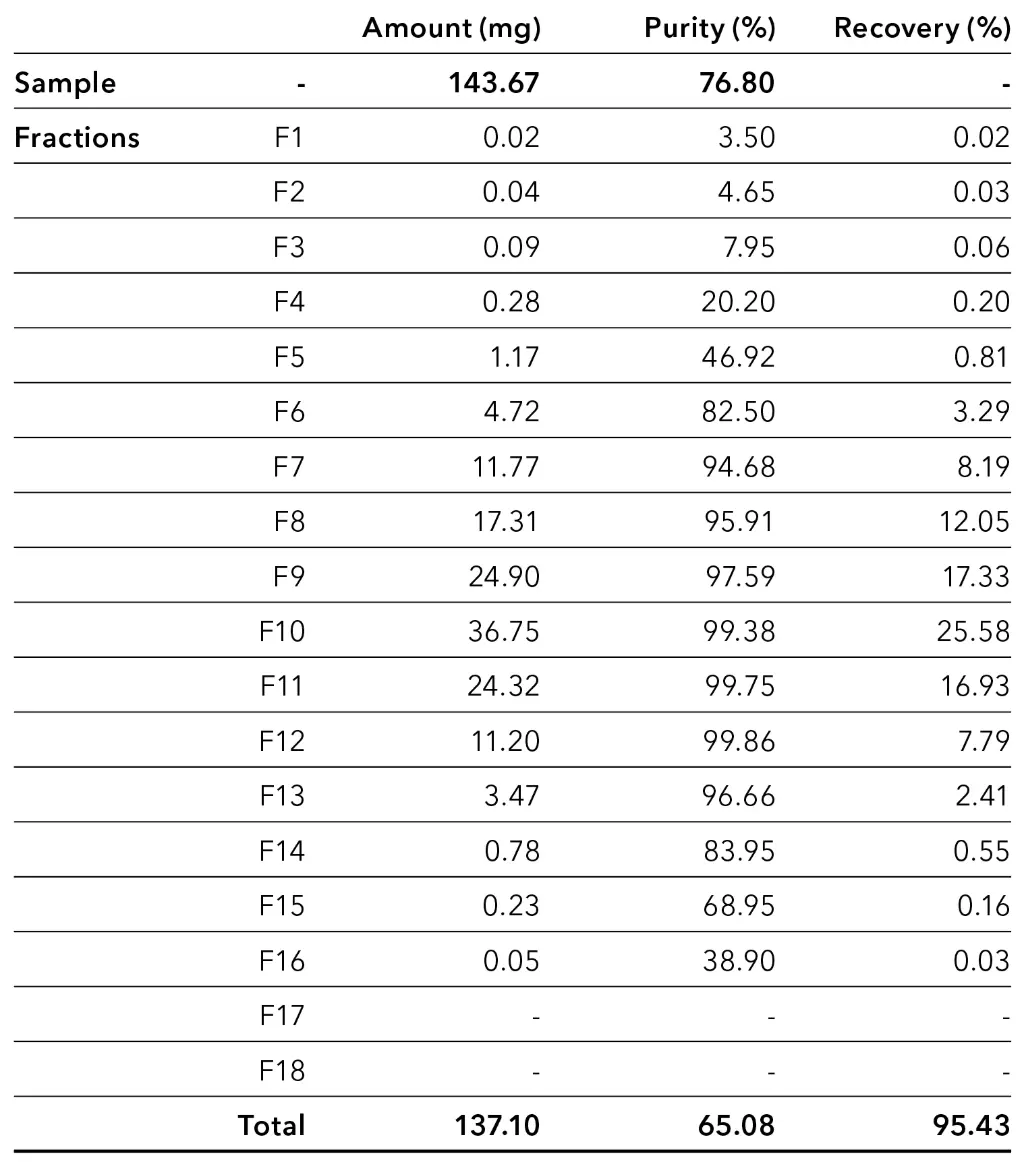
The results of the measurements with an injection volume of 4 000 µl showed that a high recovery of CBD with over 95 % was achieved. The analysis revealed that fractions 7 to 13 mainly contained CBD with a purity of approx. 95-100 %. Based on the values for CBD in each collected fraction, larger fractionation windows with theoretical values for CBD were determined and shown in Fig. 5 and Tab. 2.
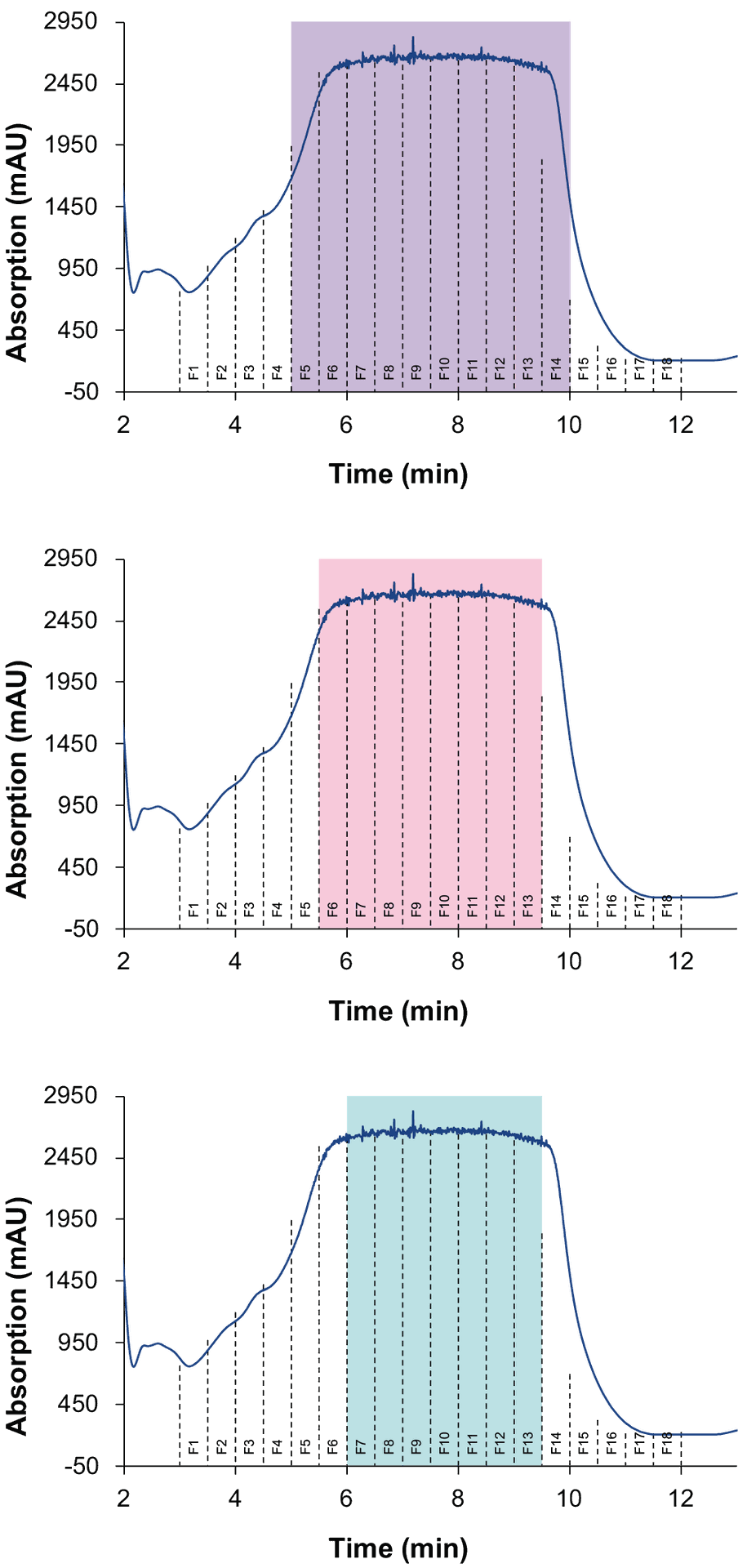
Fig. 5 Different selected fraction groups.
Tab. 2 Theoretical calculated amount, purity and recovery for CBD in selected fraction groups.
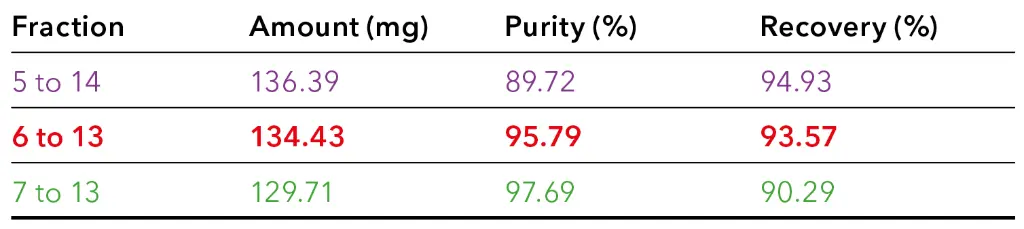
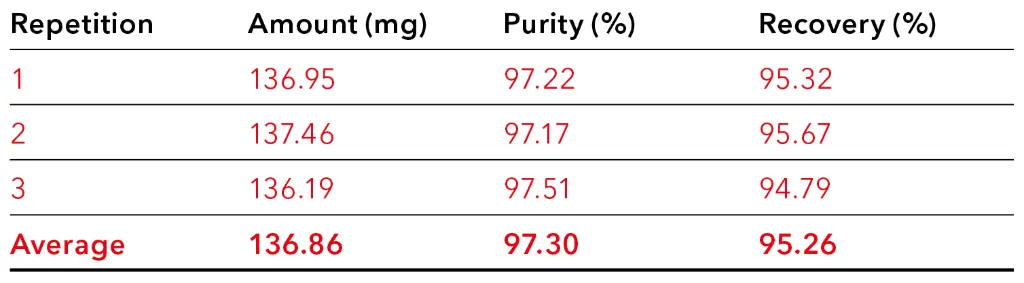
The table shows that an increased purity results in reduced recovery. For example, when fractions 7 to 13 were pooled, the purity of CBD was over 97 %, but a small amount of CBD was lost. On the other hand, pooling more fractions, such as fractions 5 to 14, resulted in the highest possible yield, but with a lower purity of CBD. The calculations showed that the best result was achieved by pooling fractions 6 to 13. Here, a high purity of approximately 96 % and a yield of about 134 mg were expected. To verify this prediction, a measurement was conducted, and the fractionation was carried out in a time window between 5.50 and 9.50 minutes. The results are shown in Tab. 3. These confirmed that the values correspond to the theoretical values. In total, approximately 137 mg of CBD with a purity of approximately 97% was purified from 4ml CBD-rich extract.
Tab. 3 Amount, purity and recovery of CBD in the selected fractions with an injection volume of 4 000 µl.
Conclusion
The analysis of the used cannabis extract showed that the main cannabinoid was CBD with a purity of 77 %. A preparative ethanol-based gradient method was developed to purify CBD from the CBD-rich extract using batch chromatography. Approximately 137 mg CBD was purified from a 4 ml sample (equals 3.4 g CBD-rich extract) with high recovery of 95 % and high purity of
97 %. The results show how a basic preparative AZURA HPLC system with a pump, a single wavelength detector and a fraction collector can be to purify high purity target compounds from a sample. The shown method is an example and was targeted at one cannabinoid. Depending on the target, the method can be adapted to other target compounds from different samples.
Material and Methods
Tab. 4 Method parameters after the scale-up.
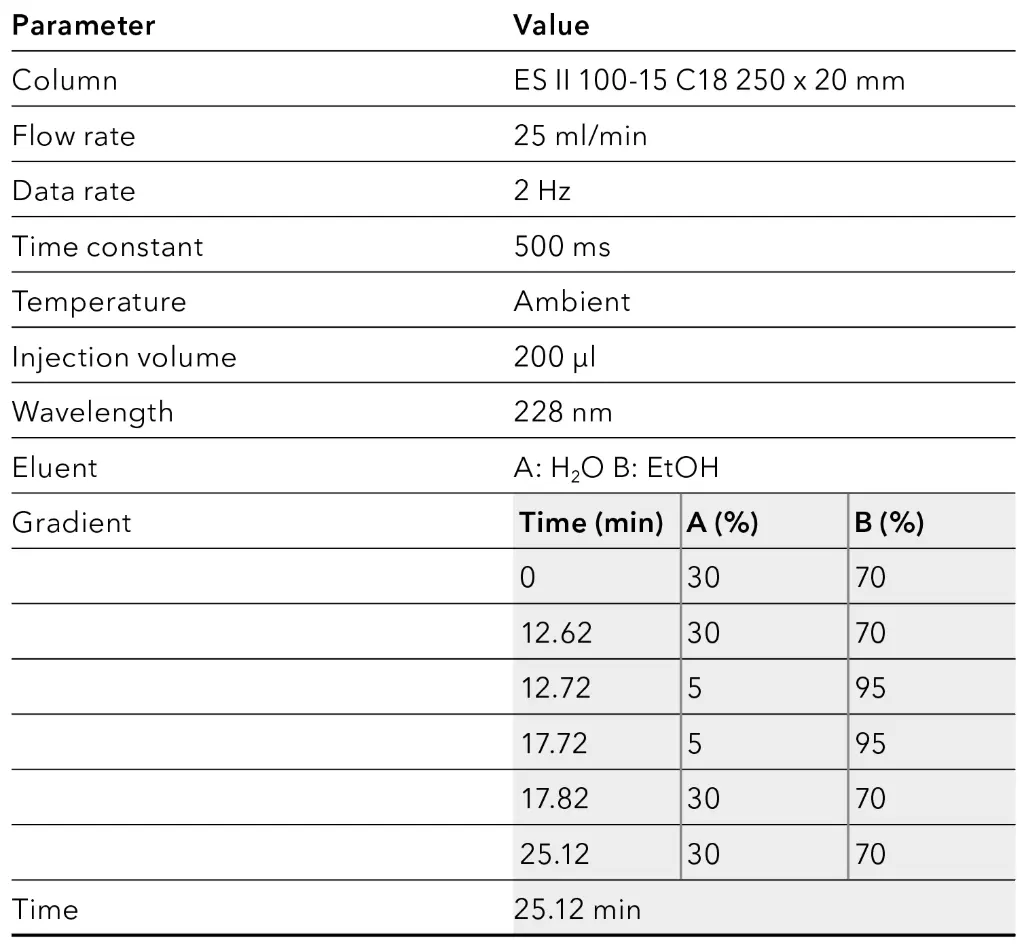
Tab. 5 Final gradient.
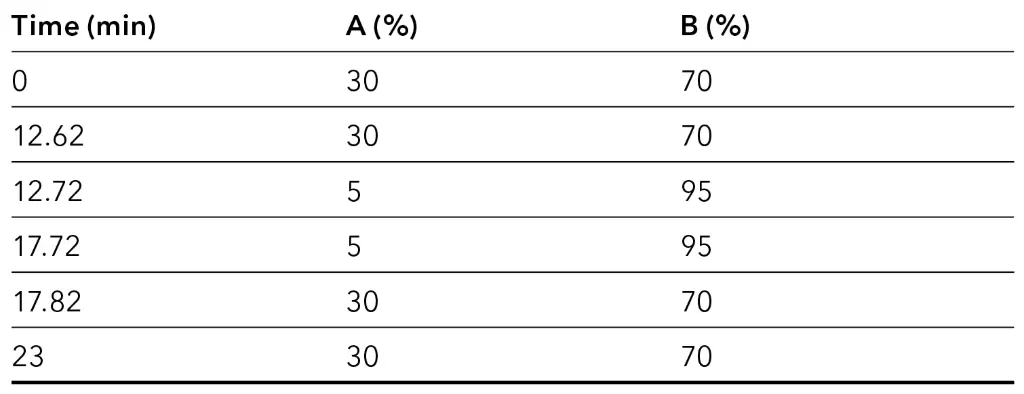
Tab. 6 Preparative system configuration.
Instrument | Description | Article No. |
Eluent tray | AZURA E 2.1L | |
Assistent | AZURA ASM 2.2L | |
Left module: UVD 2.1S | ||
Middle module: valve drive VU 4.1 | ||
Right module: P 4.1S | ||
Flow cell | Semi-preparative UV Flow cell, 3 mm path length, 1/16", 2 μl volume, 300 bar, sst | |
Injection valve | 4-position valve, 8 ports, sst, 1/16" | |
Sample pump | 10 ml pump head, sst | |
Pump | AZURA P2.1L, 250 ml pump head, sst | |
Ternary LPG module | AZURA LPG ternary module for pump P2.1L | AZZ00AB |
Column | Eurospher II 100-15 C18 250 x 20 mm | |
Capillary | 1/16", 0.45 mm, sst | |
Fraction collector | For 1/16" or 1/8" | |
Dynamic mixer | Preparative HPLC, 1/8", 5.9 ml mixing volume, 250 V, 250 bar, titanium | |
Software | Purity Chrom® 6 |

Fig. 6 System setup of preparative HPLC system; left: fraction collector; middle: mixer; right from top to bottom: eluent tray with bottles, assistant module with column holder and column, pump.
References
[1] Blanc CL et al. Purification of organic acids by chromatography: Adsorption isotherms and impact of elution flow rate. Separation and Purification Technology. 141. 2015.
[2] Pertwee RG. Handbook of Cannabis. Oxford University Press. First edition. 2014.
[3] Aizpurua-Olaizola O et al. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. Journal of Natural Products. 79. 2016.
[4] Turner CE et al. Constituents of Cannabis sativa L. XVII. A Review of the Natural Constituents. Journal of Natural Products. 43. 1980.
[5] Hazekamp A. The Trouble with CBD Oil. Medical Cannabis and Cannabinoids. 1. 2018.
[6] Perez E et al. In Vitro and Clinical Evaluation of Cannabigerol (CBG) Produced via Yeast Biosynthesis: A Cannabinoid with a Broad Range of Anti-Inflammatory and Skin Health-Boosting Properties. Molecules. 27. 2022.
[7] ElSohly MA. Marijuana and the Cannabinoids. Humana Press. 2007.
[8] Loxterkamp, L., Monks, K. (C)an(n)alyze: determination of 16 cannabinoids inside flowers, oils and seeds. KNAUER Wissenschaftliche Geräte GmbH (2020) https://www.knauer.net/en/cannalyze-determination-of-16-cannabinoids-inside-flowers-oils-and-seeds/a34489
Application details
Method | Preparative HPLC |
Mode | RP |
Substances | Cannabidiol (CBD), Cannabichromene (CBC), Tetrahydrocannabinol (THC) |
CAS number | 13956-29-1, 20675-51-8, 1972-08-3 |
Version | Application No.: VPH0080 | Version 1 07/2024 | ©KNAUER Wissenschaftliche Geräte GmbH |

