Science with Passion
Application No.: VBS0084 Version 1 07/2024
Systematic and efficient method scale-up for peptide purification
J. Menke, U. Krop, G. Greco; menke@knauer.net
KNAUER Wissenschaftliche Geräte GmbH, Hegauer Weg 38,
14163 Berlin; www.knauer.net
Summary
Peptides, crucial in therapeutic advancements, are synthesized through refined methods in biotechnology. High-performance liquid chromatography (HPLC) plays a pivotal role, ensuring the purity of synthesized peptides. Linear scale-up of HPLC methods becomes imperative for enhancing productivity and method robustness in pharmaceutical and analytical laboratories. This process involves meticulous adjustments to parameters, such as column size and solvent composition, to maintain consistent chromatographic performance. Scaling up begins with optimizing the method on a larger scale, ensuring minimal sample loss while maximizing productivity.
Introduction
Peptides, composed of short chains of amino acids, bridge the gap between small organic molecules and large proteins. They offer advantages such as specificity, selectivity, and lower immunogenicity. Recent approvals of peptide-based drugs highlight their growing significance in therapeutic areas like metabolic disease, cardiovascular health, and oncology [1].
Peptide synthesis involves creating peptide bonds between amino acids to form peptides. Solid-phase and liquid-phase methods are commonly employed, with solid-phase synthesis, introduced by Merrifield, being prominent. This process has been refined through innovations in automation and chemistry [2].
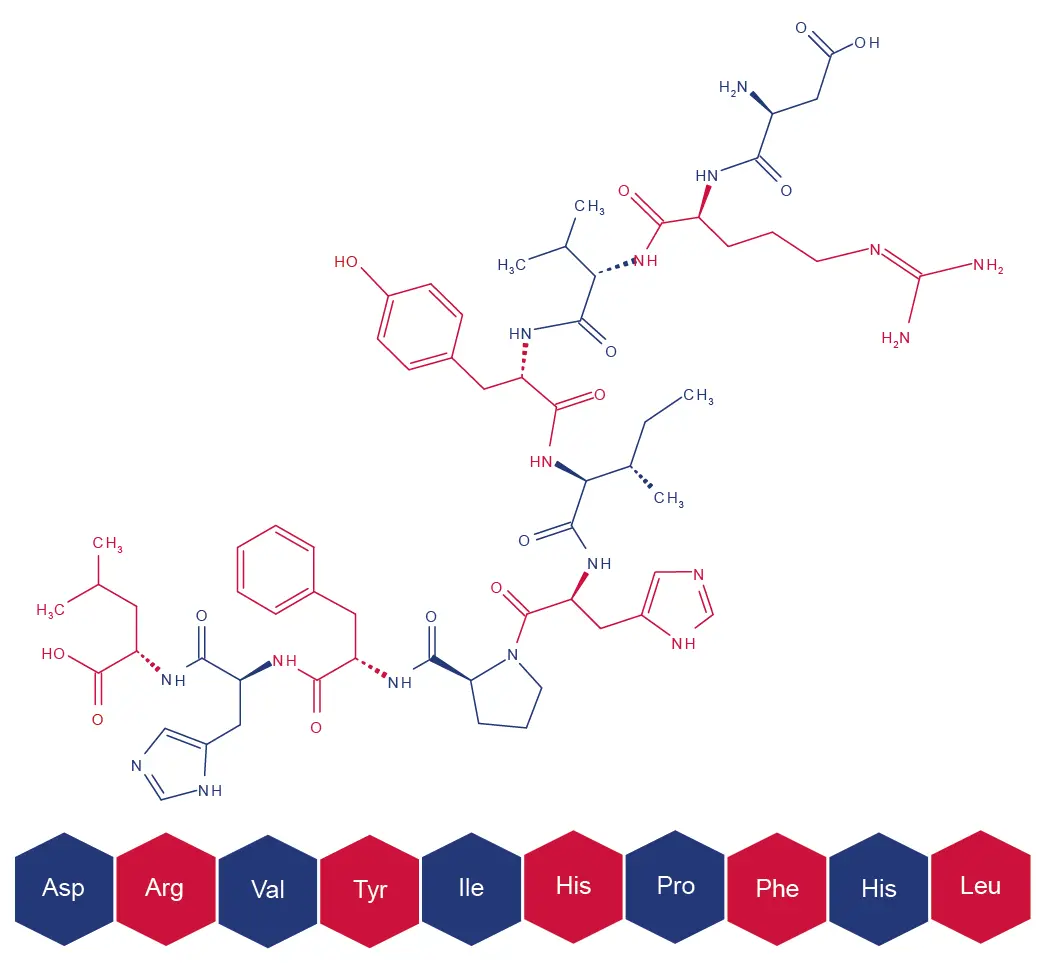
High-performance liquid chromatography (HPLC) serves both analytical and purification purposes in peptide synthesis [3]. It is applied in tracking reaction progress, verifying intermediate identities, and confirming the purity of the final peptide [3]. Here, we show a HPLC method development and method scale-up for peptide purification. As model we selected Angiotensin I, a precursor in the renin-angiotensin system, vital in blood pressure regulation, maintaining electrolyte balance and vascular tone [4].
Scaling up HPLC methods necessitates linear scale-up, where adjustments to parameters like flow rate, column size, and solvent composition maintain proportional relationships. This ensures consistent chromatographic performance with increased sample volumes, crucial for pharmaceutical and analytical laboratories striving to enhance productivity while maintaining method robustness.
For the scale-up process an optimized analytical HPLC method is the first step. Initially, the particle size of the stationary phase is adjusted to preparative scale. The method is then adjusted to the new stationary phase with the later purification in mind. The next step before shifting to preparative scale is performing a volume overload study. These experiments give insight about the target substances elution behavior and possible coeluting impurities. At last, for purification a linear scale-up is performed. All evaluated method parameters are scaled to the desired preparative column dimension. Since the scale-up is linear, elution is predictable, and purification can start without losing significant amounts of sample for method evaluation.
Sample Preparation
The crude Angiotensin I sample was commercially obtained (ProteoGenix SAS, Schiltigheim, France) as lyophilized powder with a nominal purity of 68.05 %. The crude was reconstituted in 25:75 acetonitrile (gradient grade)/distilled water (v:v). A solution with a concentration of 1 mg/ml was prepared. The solution was filtrated using a syringe filter with a 0.45 µm regenerated cellulose membrane (RC).
Results
The crude peptide was measured using an analytical HPLC system (see Fig. 1). A method with an overview gradient from 10 to 100 % organic eluent was applied. One large peak was identified as Angiotensin I. Several neighboring impurities were observed. The purity of the crude Angiotensin I was confirmed to be about 68.05 % (target peak area relative to impurities peak areas).
As a first step for scale-up of an analytical method, the particle size was increased from 5 to 10 µm (see Fig. 2), to mitigate the increase in backpressure at higher flow rates in preparative scale. A focused gradient was applied to increase resolution of Angiotensin I and the neighboring impurities (see Fig. 3). The method was shortened, introducing a wash step with 100 % organic eluent into the gradient, followed by an equilibration step to starting conditions. This removed all late eluting impurities and shortened the overall method runtime.
With an optimized method a volume overload study was performed to gather insight of the target peaks elution behavior (see Fig. 4). It was observed that above 50 µl injection volume, separation of impurities and target substance was poor. Therefore 50 µl was taken as calculation base for scale-up. Since all experiments were in analytical scale, sample loss could be kept minimal. The next step in the linear scale-up workflow was the increase of the column inner diameter (ID) from 4 to 20 mm. Using the KNAUER HPLC Method Converter, preparative scale method parameters were calculated (see Fig. 5) (https://www.knauer.net/software-hplc-method-converter).
Finally, the method was transferred into preparative scale and to a preparative HPLC system. 2000 µl of crude sample with a concentration of 1 mg/ml were injected. The target peak eluted as predicted and was fractionated with the first run already. Fraction slices of 2.5 ml were chosen.
The target fractions were pooled and analyzed using the original analytical HPLC method confirming a purity of > 99 % (see Fig. 6). That results in 1.4 mg of purified peptide per run.
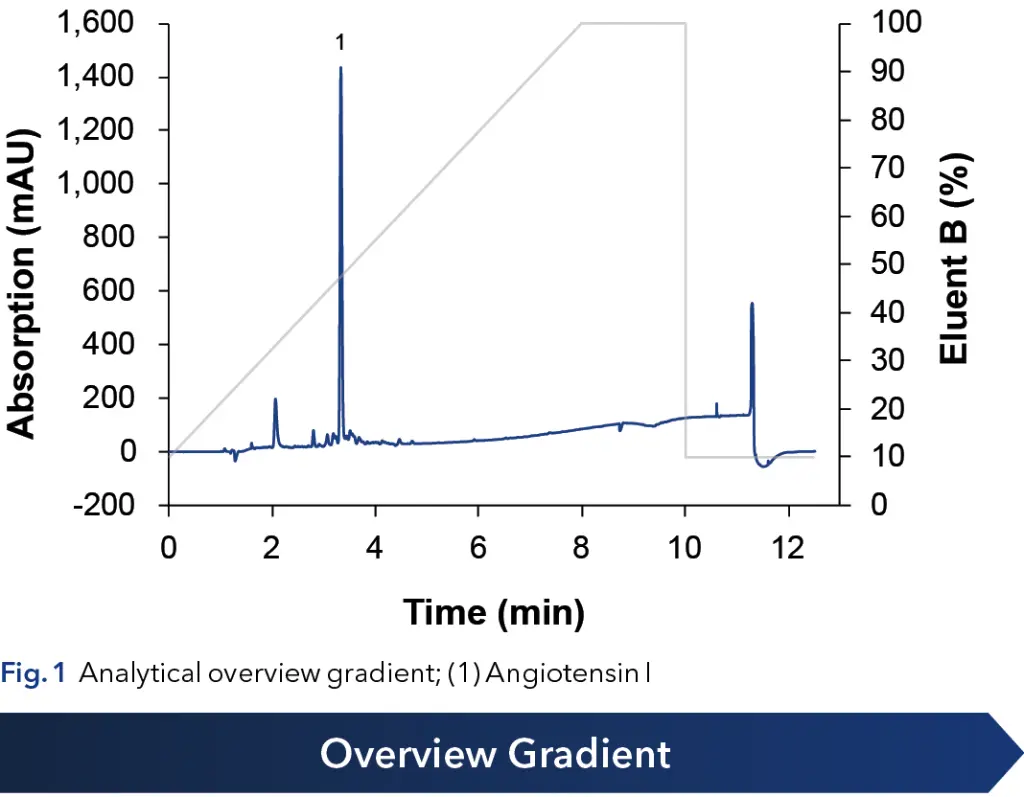
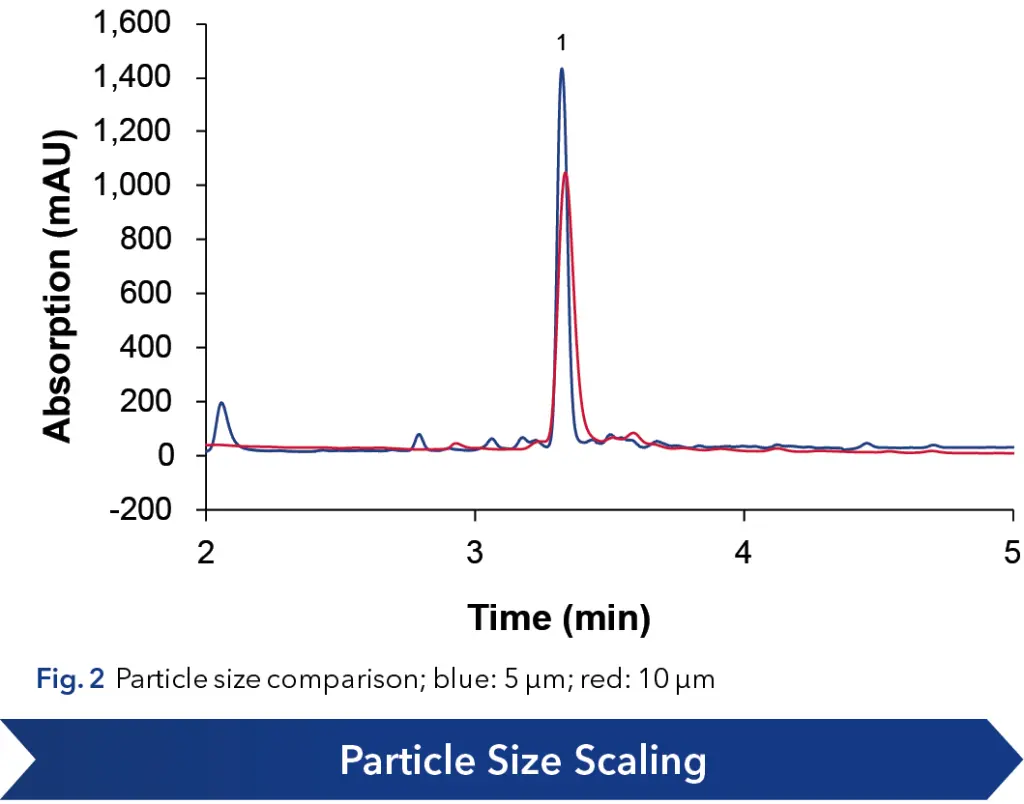
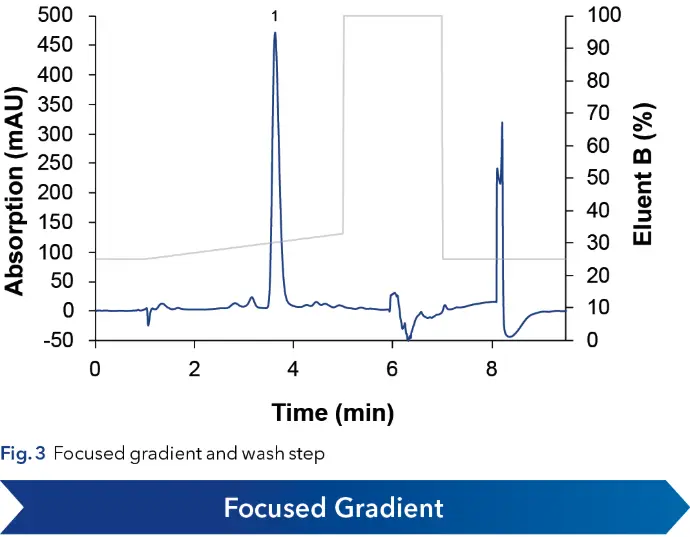
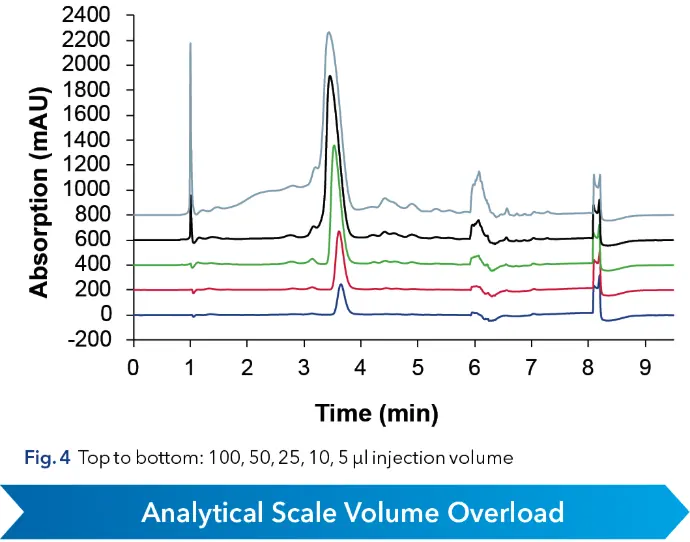
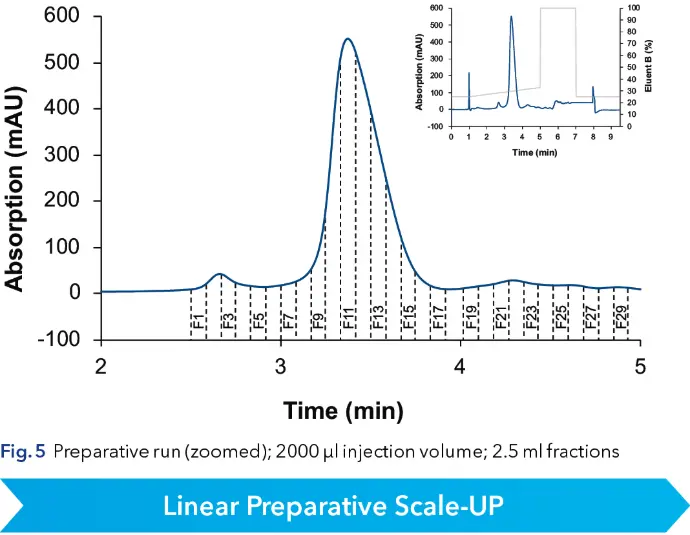
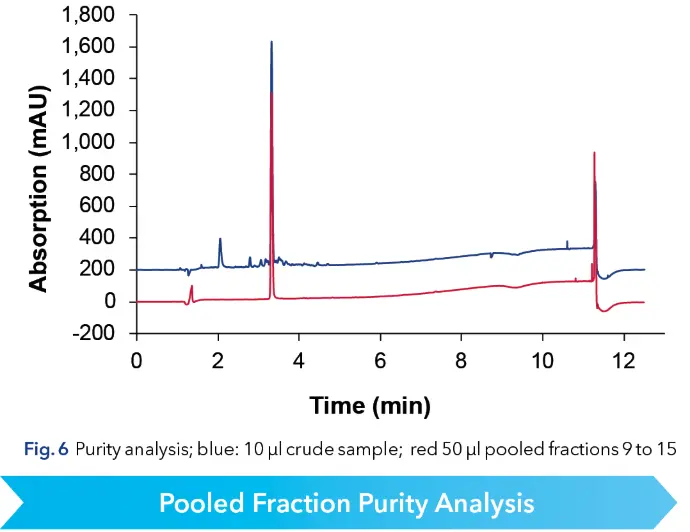
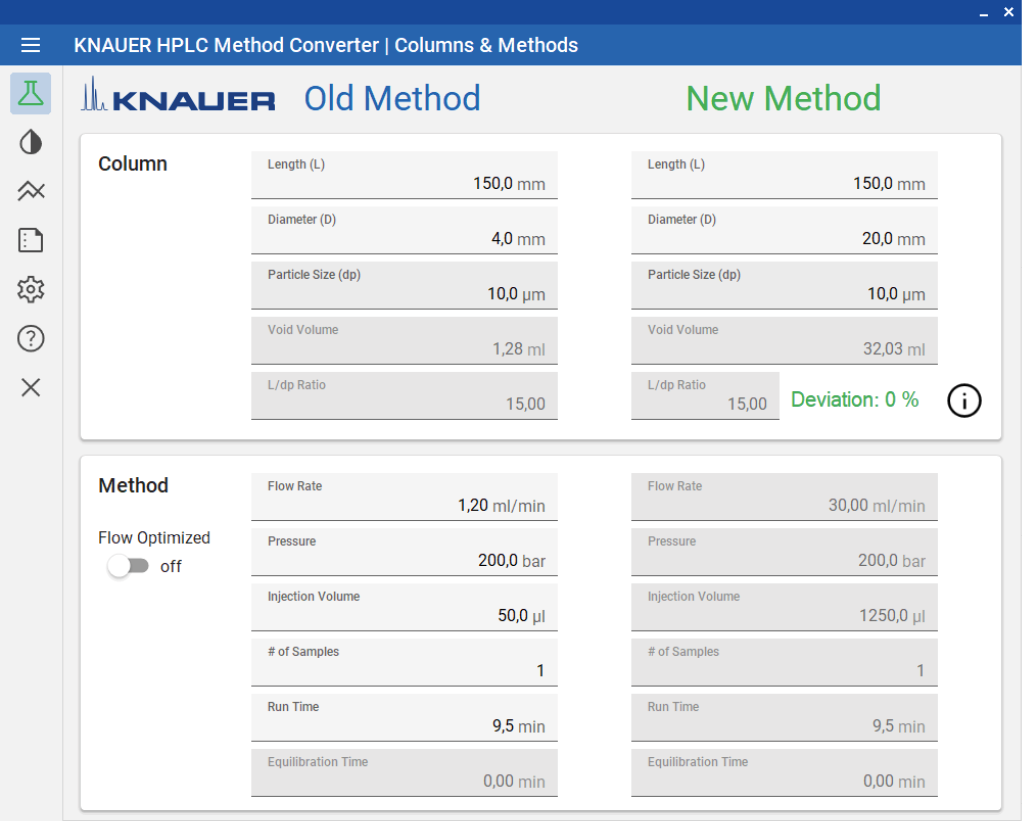
Fig. 7 Method scale-up with the “KNAUER HPLC Method Converter”
DOWNLOAD HPLC METHOD CONVERTER
Fig. 8 Angiotensin Structure
Conclusion
In conclusion, the linear scale-up of the analytical HPLC method for purifying crude Angiotensin I proved effective in enhancing the purity of the target peptide. By systematically increasing the particle size and adjusting method parameters, such as the gradient and injection volume, the method was optimized for preparative scale. The successful transfer to a preparative HPLC system, guided by linear scale-up principles, enabled efficient fractionation of the target peptide with high purity (>99%) achieved in the pooled fractions. This underscores the importance of linear scale-up in maintaining method robustness and ensuring the successful purification of peptides at a larger scale.
Material and Methods
Tab. 1 Overview Gradient
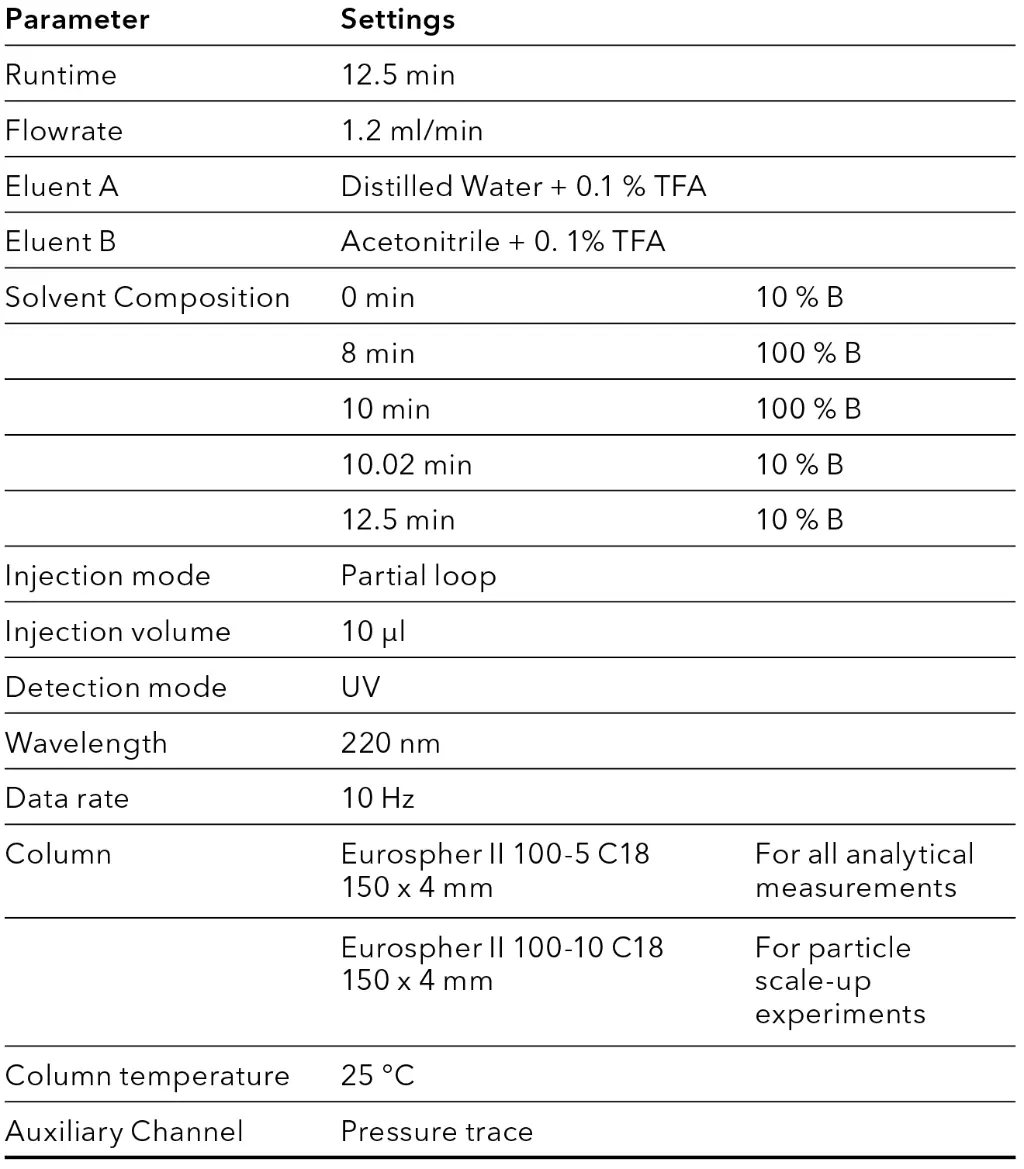
Tab. 2 Focused Gradient
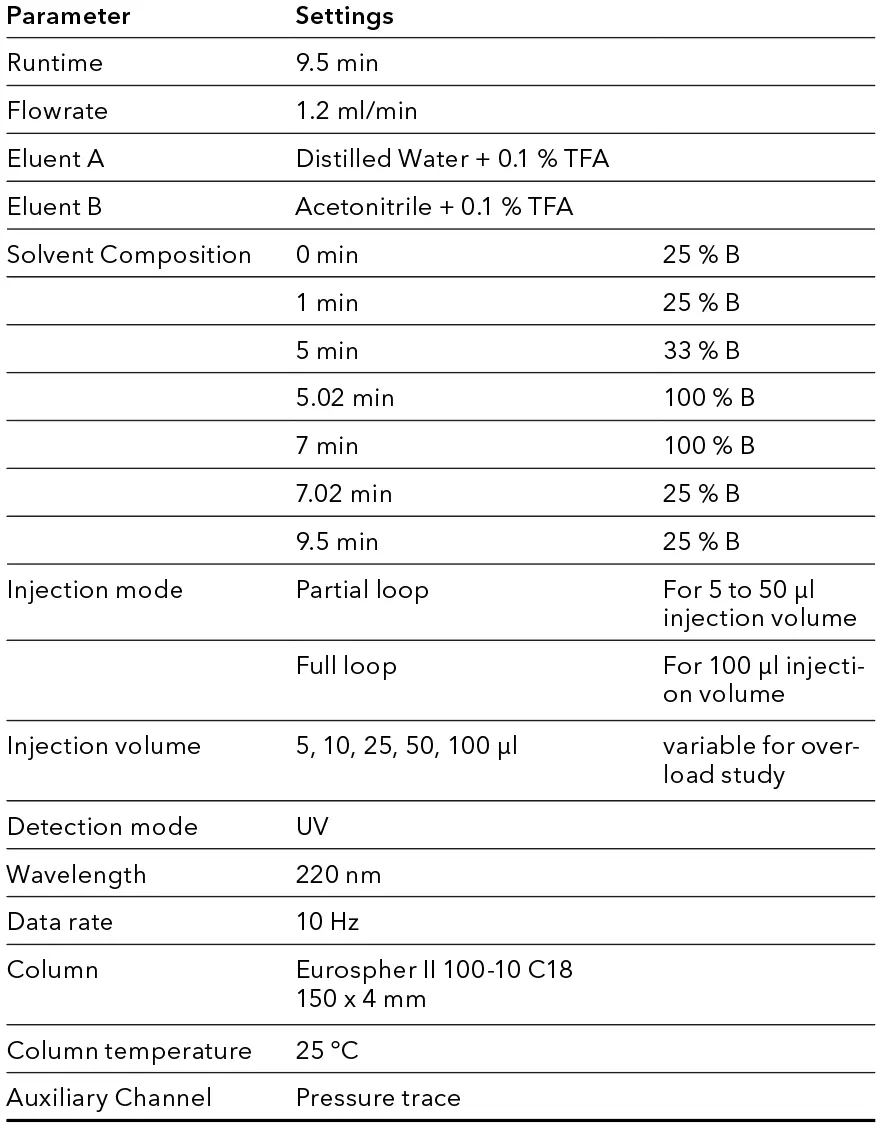
Tab. 3 Preparative Method
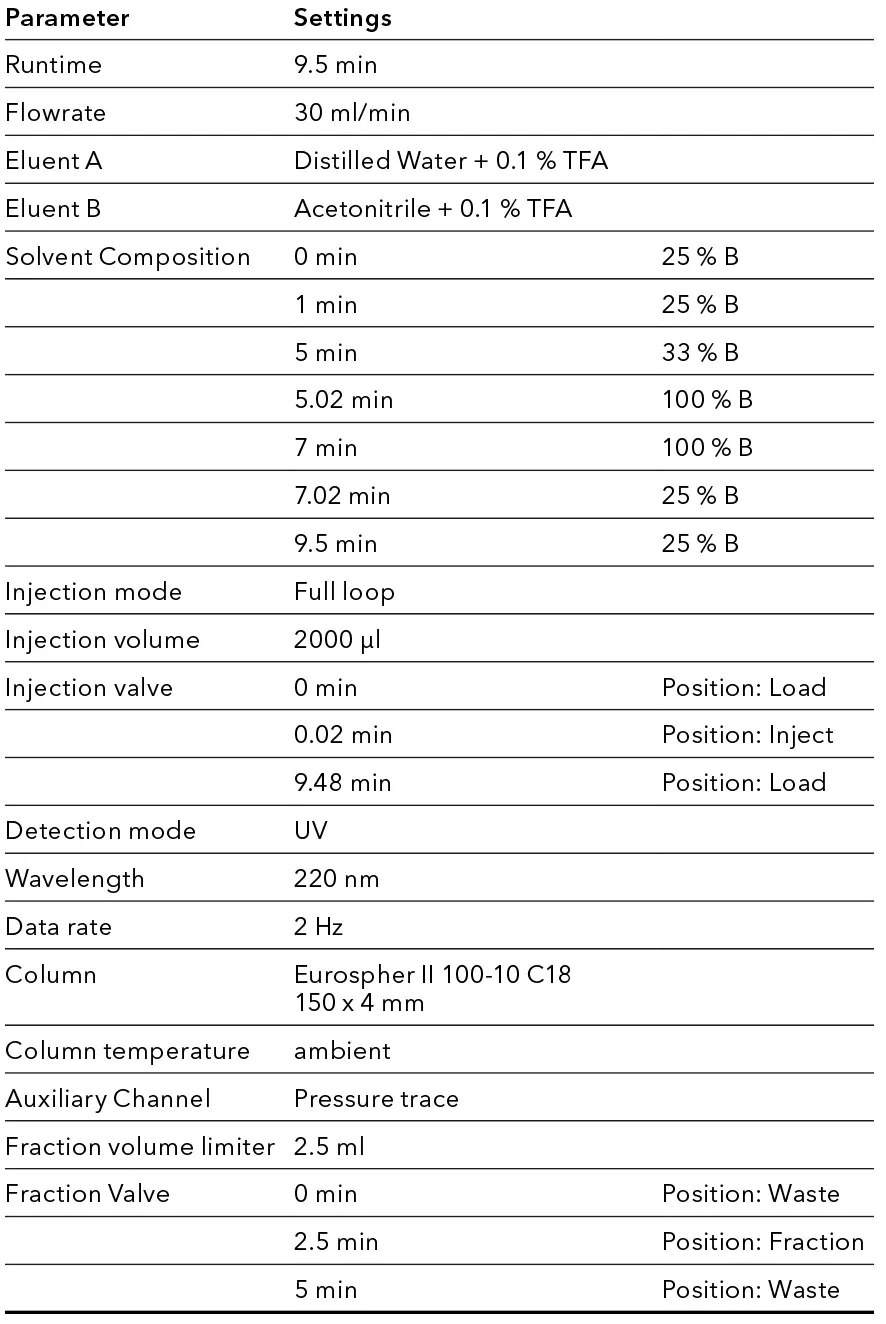
Tab. 4 AZURA® analytical HPLC System
|
Instrument |
Description |
Article No. |
|
Eluent tray |
AZURA Eluent tray E 2.1L |
ACZ00 |
|
Pump |
AZURA P 8.1L |
|
|
Autosampler |
AZURA AS 6.1L |
|
|
Column thermostat |
AZURA CT 2.1 |
|
|
Detector |
AZURA DAD 2.1L |
|
|
Measuring cell |
Standard KNAUER LightGuide UV Flow Cell Cartridge 10 mm path length, 1/16", 2 µl volume, 50 bar |
|
|
Capiliaries |
AZURA Analytical K-Connect start-up kit, stainless steel, 1/32" capillaries with fitting sleeves for 1/16"connections 0.1 mm ID |
|
|
Mixer |
HPLC eluent mixer, stainless steel, 200 µl |
|
|
Column 1 |
Eurospher II 100-5 C18 150 x 4 mm |
|
|
Column 2 |
Eurospher II 100-10 C18 150 x 4 mm |
|
|
Software |
ClarityChrom® 9.0 Workstation, autosampler control included |
|
|
Software |
ClarityChrom® 9.0 PDA extension |

Tab. 5 AZURA® semi-preparative System
|
Instrument |
Description |
Article No. |
|
Eluent tray |
AZURA Eluent tray E 2.1L |
ACZ00 |
|
Pump |
AZURA P 6.1L High Pressure Pump with 50 ml pump head, stainless steel, without Degasser |
|
|
Assistant |
AZURA Assistant ASM 2.2L |
AY01403 |
|
|
Left module: UV detector UVD 2.1S |
EDA03XA |
|
|
Middle module: Empty module |
|
|
|
Right module: Valve drive VU 4.1 |
EWA04 |
|
Valve |
AZURA V 4.1 Valve 2-position valve with 6 ports, 1/16" |
|
|
Flow Cell |
Semi-preparative UV Flow Cell 3 mm path length, 1/16", 2 µl volume, 300 bar, stainless steel |
|
|
Fraction Collector |
Fraction Collector Foxy R1 |
|
|
Mixer |
HPLC eluent mixer, stainless steel, 600 µl |
|
|
Column |
Eurospher II 100-10 C18 150 x 20 mm |
|
|
Software |
PurityChrom® 6 Basic License |

References
[1] Vora, D., Dandekar, A. A., Banga, A. K. In Peptide Therapeutics: Fundamentals of Design, Development, and Delivery, Jois, S. D., Ed., Springer International Publishing, Cham, 2022, pp. 183–201.
[2] Bodanszky, M. Principles of Peptide Synthesis, Springer, Berlin, Heidelberg, 1993.
[3] Mant, C. T., Chen, Y., Yan, Z., Popa, T. V., Kovacs, J. M., Mills, J. B., Tripet, B. P., Hodges, R. S. In Peptide Characterization and Application Protocols, Fields, G. B., Ed., Humana Press, Totowa, NJ, 2007, pp. 3–55.
[4] Reid, I. A. Advances in Physiology Education 1998, 275, S236-245.
Application details
Method | Preparative HPLC |
Mode | RP |
Substances | Angiotensin I |
CAS number | 484-42-4; 9041-90-1 |