Science with Passion
Application No.: VBS0082 Version 1 10/2023
Large scale purification of oligonucleotides with ion exchange chromatography
U. Krop1, T. Pöhlmann2, N. Schneider2; krop@knauer.net
1 KNAUER Wissenschaftliche Geräte GmbH, Hegauer Weg 38,
14163 Berlin; www.knauer.net
2 BianoGMP GmbH, Ronneburger Straße 74, 07546 Gera, Germany


Summary
The field of oligonucleotide therapeutics has made remarkable progress in recent years leading to a growing need for purified oligonucleotides. One of the classic oligonucleotide production techniques is chemical synthesis followed by a chromatographic purification step. Especially for up scaling, ion exchange chromatography is the ideal method due to its high loading capacity combined with high resolution and yield. Here we present the work of BianoGMP GmbH. The company specialised in the production of high purity and quality oligonucleotides in quantities of up to several grams for research and development and clinical programmes. BianoGMP GmbH has many years of experience in the development of therapeutic oligonucleotides with a focus on GMP services and manufacturing process optimisation. Here we demonstrate a successful large-scale ion exchange chromatography purification of an oligo dT with 95 % purity and 90 % yield.
Introduction
During the last two decades oligonucleotides have become an important tool in basic research and a potent technology for drug development. Especially silencing oligonucleotides, such as siRNA and antisense DNA, stepped into clinical development and the first oligonucleotide drugs have been approved by the FDA (e.g. Patisiran) in the last years[1]. These oligonucleotide-based drugs proved their therapeutic potency and showed an excellent toxicology profile. A major challenge during drug development is the production of GMP material, scale-up and process development. One aspect in the manufacturing process is the purification of the active pharmaceutical ingredient (API) and its separation from unwanted side products.
Chromatography is a widely used separation technique for (bio)molecules in the pharmaceutical industry that is selective, versatile, and easily scalable. The two most efficient HPLC purification methods are ion pairing reversed phase (IP-RP) chromatography or ion exchange chromatography (IEX). IP-RP uses an ion-pairing reagent to mask the charge on the molecule, that would prevent retention on a classical RP column. However, the low loading capacity is one of the drawbacks of this technique and makes it unfavourable for upscaling[2]. IEX is widely used since it is an efficient method regarding loading and separation. Furthermore, IEX can be scaled up and transferred from method development to preparative as well as commercial production equipment. IEX uses mostly aqueous buffers under denaturating conditions for DNA and non-denaturating conditions for RNA, solvents that are more environmentally sustainable in comparison to the solvents used in IP-RP. The stability of the material allows fast and high throughput separations, as well as easy handling of the resin for the regeneration and repacking of the column. One of the biggest benefits is the high loading capacity preserving high resolution and high yield. Here we describe the large-scale purification of oligo (dT) with IEX. Oligo(dT) binds to the polyA tail of mRNA and is a versatile tool often used for the isolation of mRNA or as primer for cDNA synthesis.
Experimental: Sample Preparation and Results
The oligonucleotide was synthesized and the data provided by the company BianoGMP GmbH. After solid phase synthesis the oligonucleotide is cleaved off the support and deprotected by removing remaining protection groups by incubating with ammonia. To increase stability and solubility of the sample, the oligonucleotide is subsequently neutralized with acetic acid and diluted with water to a conductivity below 30 mS/cm. Additionally, the concentration was determined by photometric measurement. HPLC analysis confirmed (Fig. 1) 89.1 % purity of the sample before the chromatographic purification. The target purity for the oligonucleotide was 95 %.
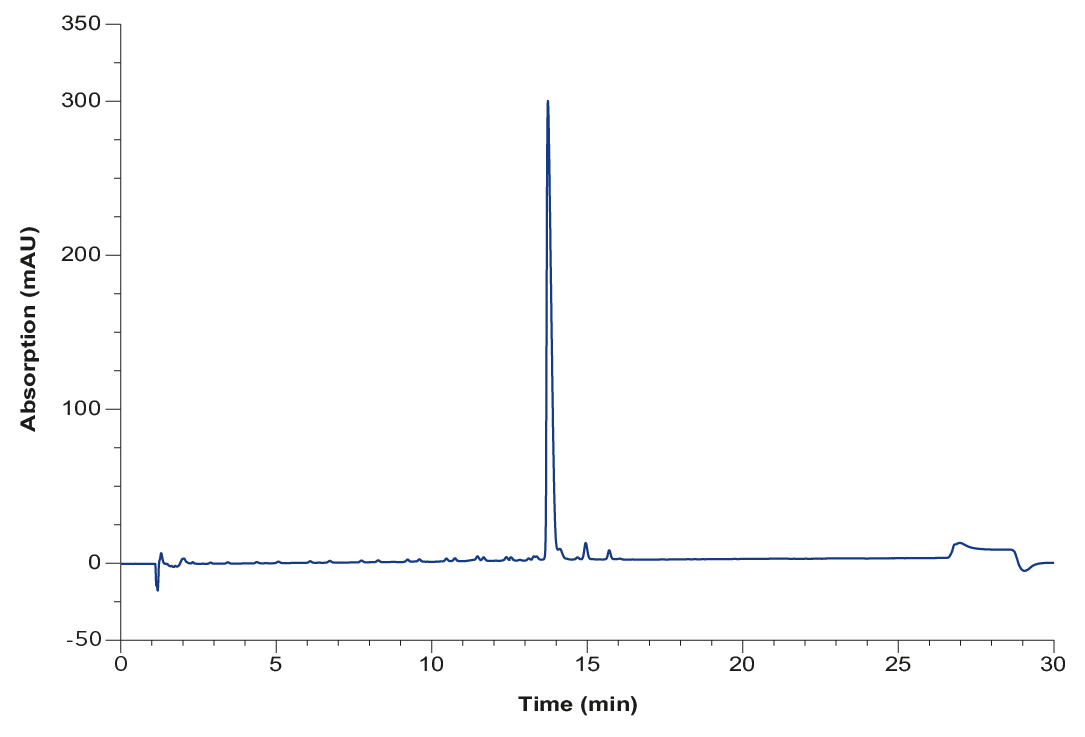
Fig. 1 Analytical chromatogram of synthesized oligo(dT) before IEX purification
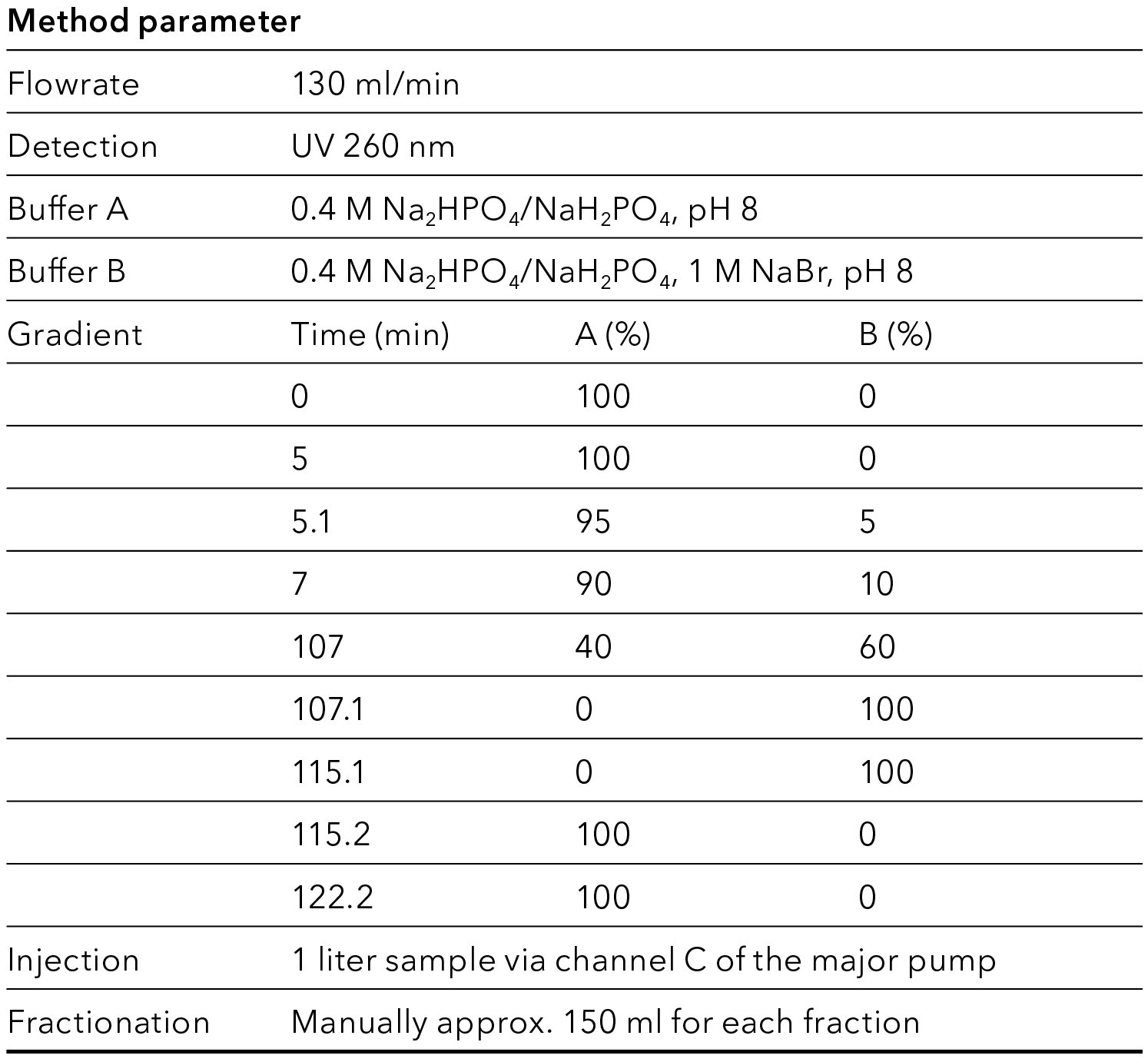
The crude oligonucleotide is further purified using preparative HPLC. The easy to use AZURA® Prep System was used for the purification. The system was equipped with a ternary preparative pump that allowed flow rates up to 220 ml/min and precise gradient formation. Purification was monitored by a single UV detector, which can also be used to trigger fractionation via the fraction valve. As the sample volume is very large for these separations, the sample was applied via the third channel of the gradient pump. Ion exchange resins are well known for very high loading capacities, while retaining high resolution and high yield. For this reason, a strong anion exchanger was chosen for the purification. The selection of buffer parameters depends on oligo specification like length and modifications. A non-denaturating phosphate buffer and sodium bromide as counterion was used for the oligonucleotide purification. Fig. 2 shows the chromatogram during gradient elution. To increase the throughput, a high loading is performed, resulting in a saturated UV signal. The elution peak was fractionated manually, and the fractions were analyzed for the purity of the oligonucleotide. The addition of a fraction collector or fractionation valve to automize the collection of purified samples is possible. Pooling of fractions resulted in 95 % purity and a yield of 90 % as shown by analytical HPLC (Fig. 3).
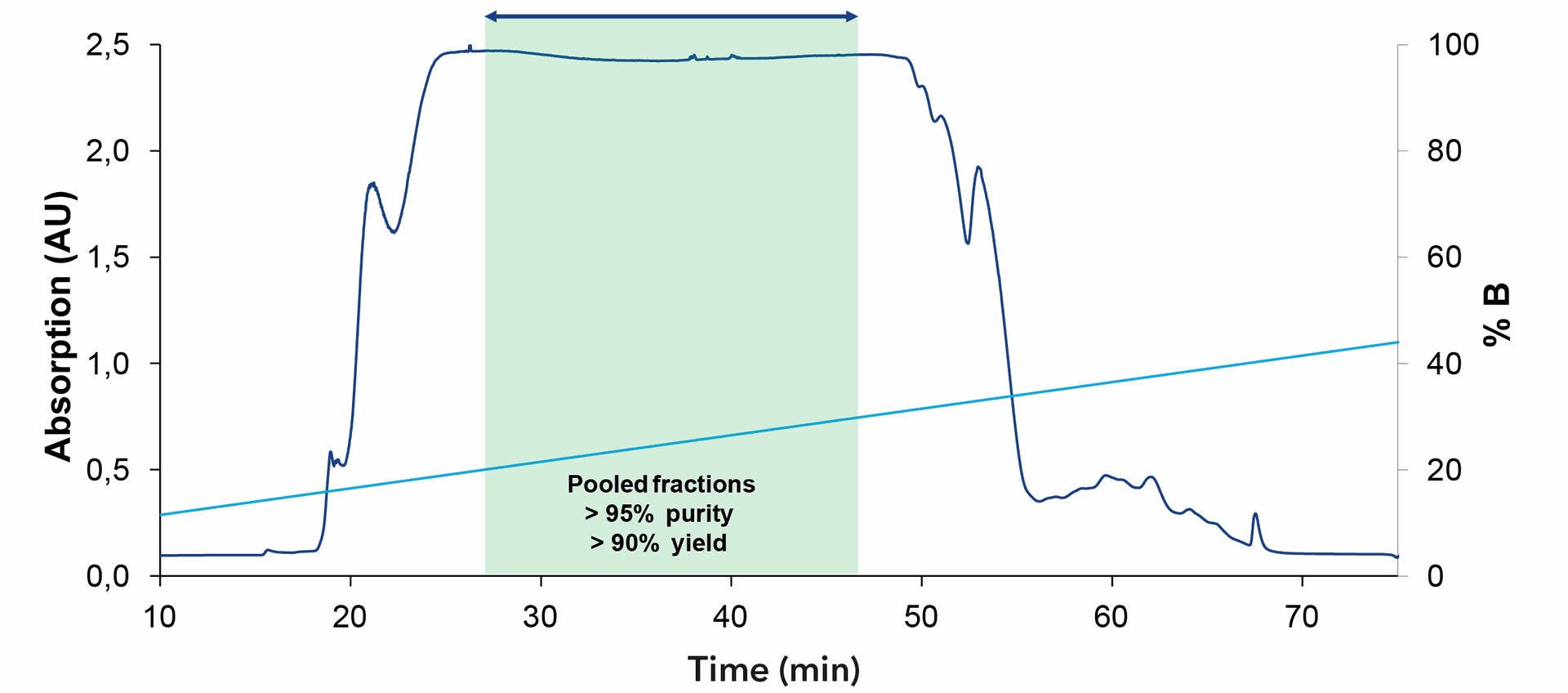
Fig. 2 Chromatogram of oligonucleotide purification from 1 l sample
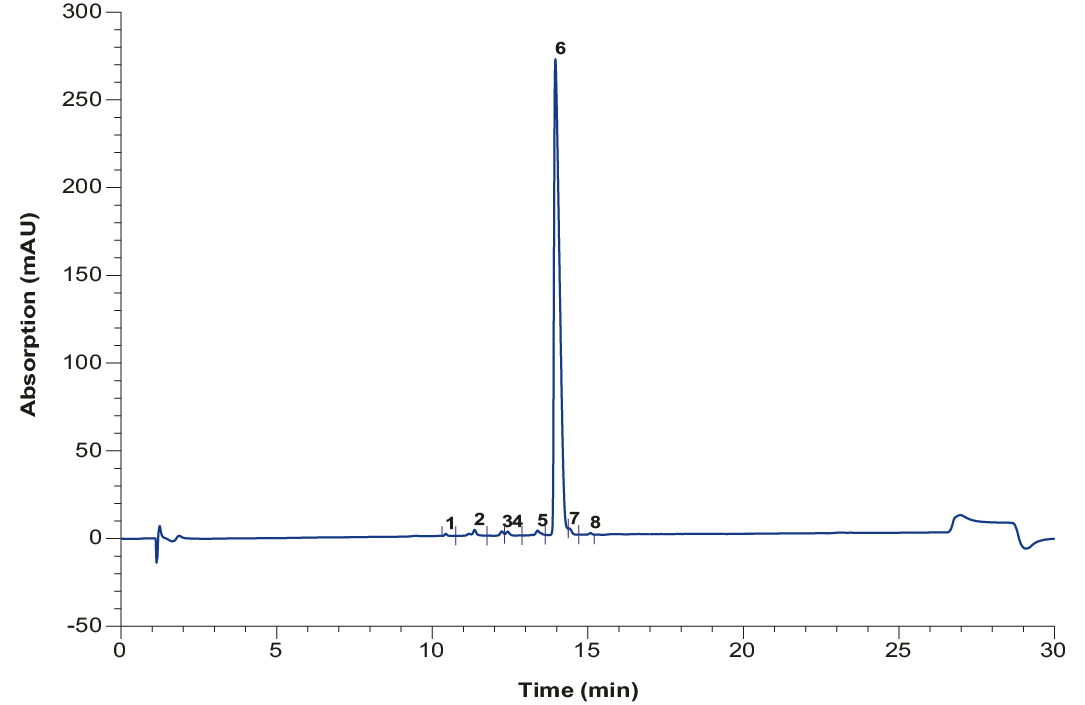
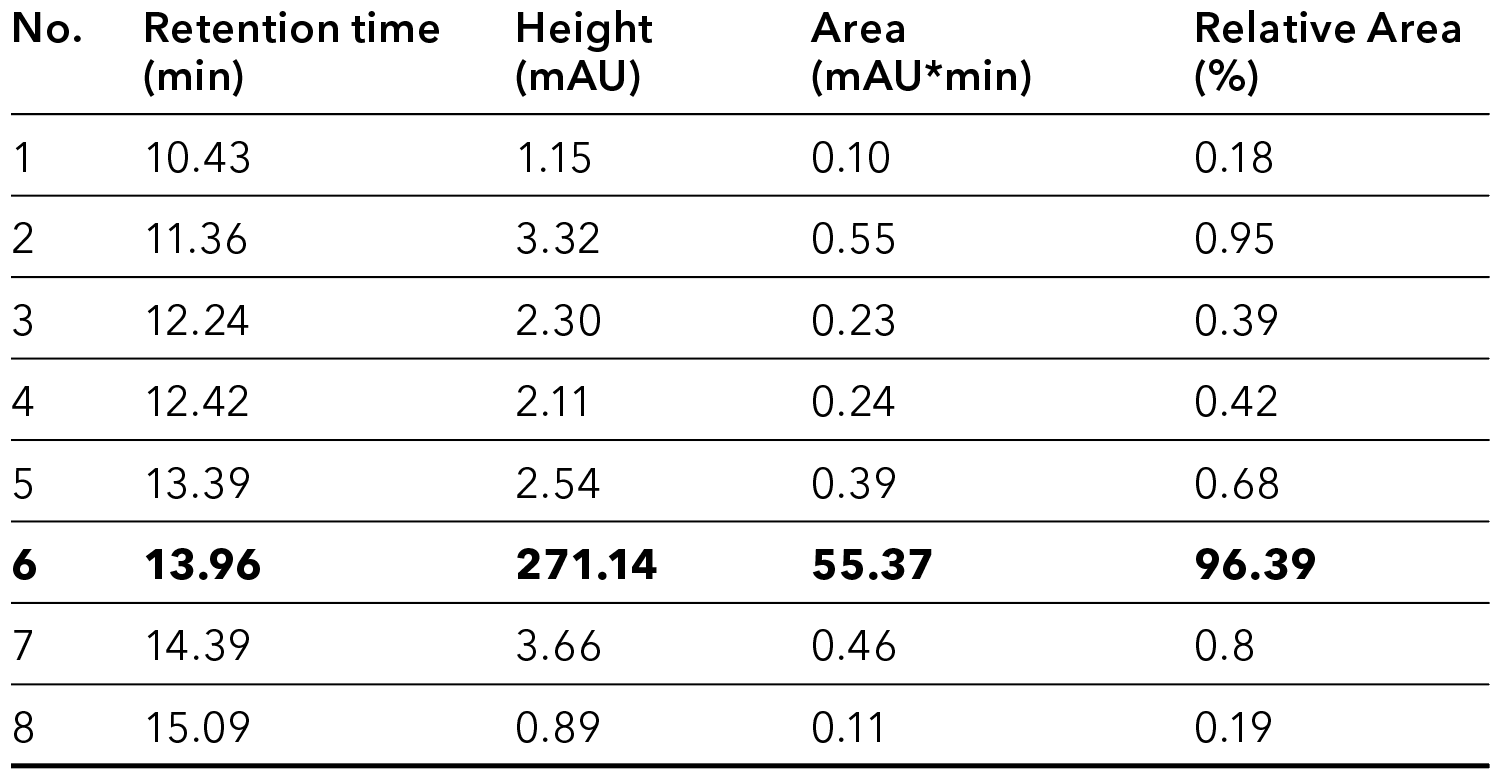
Fig. 3 Analytical chromatogram of purified and pooled oligo(dT) after IEX
Tab. 1 Peak table of analyzed pooled sample
Conclusion
Oligonucleotide-based drugs have high therapeutic potential, but their production can be challenging. There is a need for chromatographic process development and up-scaling. Ion exchange chromatography is a potent method for the purification process of oligonucleotides due to its high separation efficiency, easy handling, process stability and scalability. We showed a successful large scale ion exchange chromatography purification of an oligo(dT) with 95 % purity and 90 % yield.
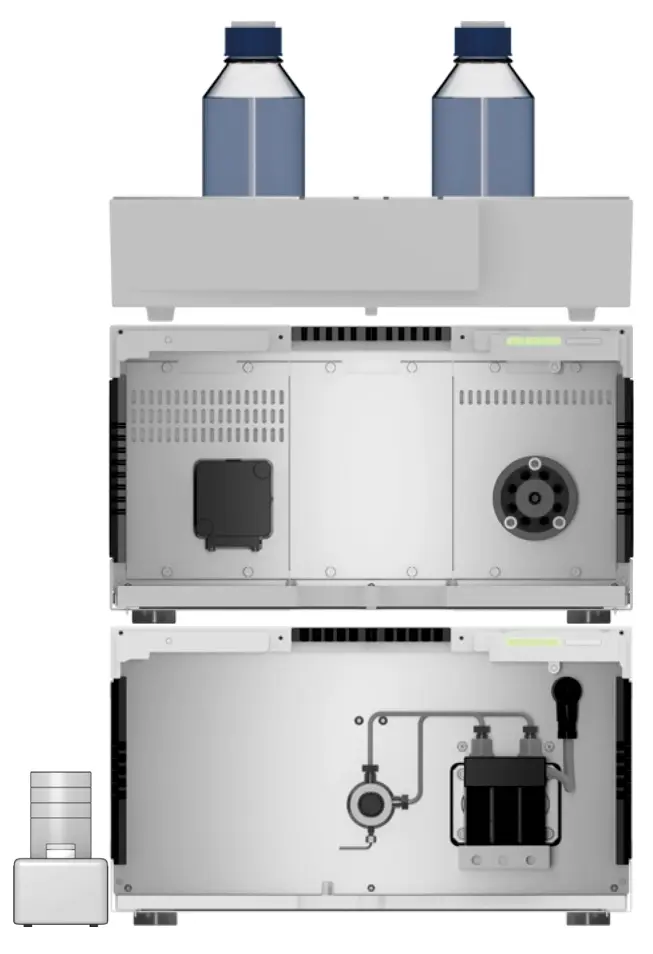
Fig. 4 Used AZURA preparative HPLC system
Material and Methods
Tab. 2 System configuration
|
Instrument |
Description |
Article No. |
|
P 2.1L
|
Preparative HPLC pump with 250 ml/min pump head, stainless steel
|
|
|
AZURA LPG ternary module
|
Ternary LPG module for flow rates up to 220 ml/min (stainless steel)
|
|
|
ASM 2.2L
|
AZURA Assistant Customizable docking station for pumps, valves, and detectors
|
|
|
|
Left module: UV detector UVD 2.1S |
|
|
|
Middle module: empty module
|
|
|
|
Right module: Valve drive VU 4.1
|
|
|
Flow cell
|
Preparative UV Flow Cell, 1/8"
|
|
Valve
| Multiposition valve, 8 Port for fractionation
|
|
Dynamic mixer
| Dynamic mixer, for preparative HPLC, stainless steel, 1/8"
|
|
Software
| ClarityChrom® with FRC Control Modul
|
|
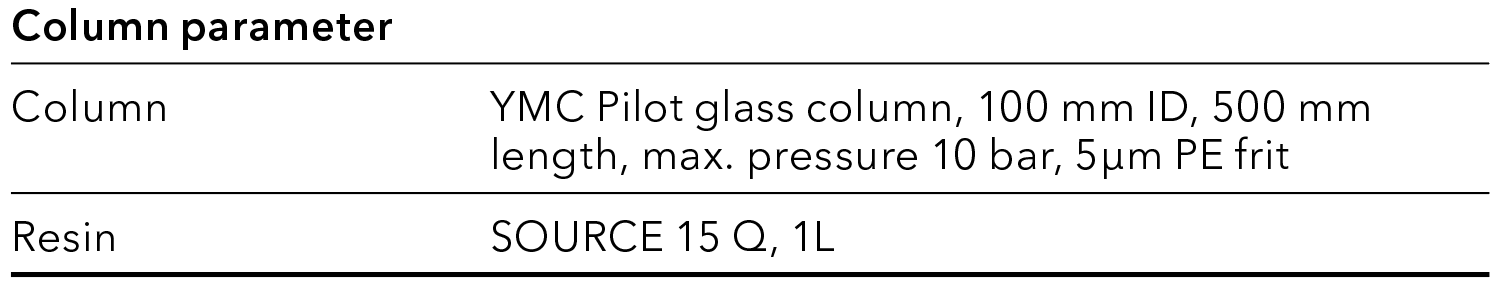
Tab. 3 Preparative column parameter
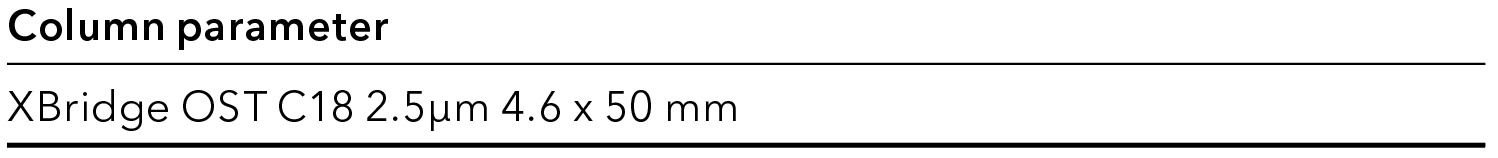
Tab. 4 Analytical column parameter
Tab. 5 Parameter for preparative method
References
[1] Wood, H. FDA approves patisiran to treat hereditary transthyretin amyloidosis. Nat Rev Neurol 14, 570 (2018).
https://doi.org/10.1038/s41582-018-0065-0
[2] Minkner, R., Boonyakida, J., Park, E. Y., & Wätzig, H. (2022). Oligonucleotide separation techniques for purification and analysis: What can we learn for today's tasks?. Electrophoresis, 43(23-24), 2402–2427.
https://doi.org/10.1002/elps.202200079
Application details
Method | HPLC purification |
Mode | IEC |
Substances | Oligonucleotides |

