Science with Passion
Application No.: VPH0077 Version 1 / 11/2023
Two-step CBG purification – combined matrix removal and peak recycling
J. Wesolowski, Y. Krauke, G. Greco; krauke@knauer.net
KNAUER Wissenschaftliche Geräte GmbH, Hegauer Weg 38, 14163 Berlin

Photo: Adobe Stock
Summary
Recycling chromatography is a powerful technique that allows the separation of two closely eluting peaks by increasing the column bed length. The technique allows the eluted substances to be recycled after passing through the column. Thereby, critical peak pairs that elute together can be separated further with each cycle. A previously presented recycling system was upgraded by adding, two additional valves. This optimization enables 1) peak parking for removal of impurities from the target compounds and 2) re-injection of the peak pair back into the system for peak recycling. The versatility of the recycling system is greatly enhanced by this upgrade. In this application, the optimized recycling system was used to remove matrix components from CBG-rich cannabis extract, allowing subsequent separation of CBG (cannabigerol) and CBD (cannabidiol) for purification of CBG. By combining two separation techniques in one system, CBG was purified to a high degree of purity, increasing from 81 % to 100 %.
Introduction
Due to the progressive decriminalization of cannabis for recreational and medicinal use, the properties of this controversial plant have received increasing attention. To date, more than 500 phytochemicals have been identified in Cannabis sativa L. These include the medically important cannabinoids but also terpenes, flavonoids, and other non-cannabinoid constituents. Cannabinoids are a group of terpenophenol compounds with a characteristic C21 framework (or C22 when in acidic form) that are responsible for many therapeutic effects. Two of the most well-known cannabinoids, beside THC (Δ⁹-trans-tetrahydrocannabinol), are CBD (cannabidiol) and CBG (cannabigerol). CBD has gained a lot of attention in recent years due to its potential health benefits.
It is often used to relieve pain, inflammation, and anxiety. CBG is less popular than CBD but is becoming increasingly important. It is often referred to as the “mother of all cannabinoids” as it serves as a precursor for other cannabinoids such as CBD and THC. Like CBD, CBG offers a variety of potential health benefits. For example, CBG was demonstrated to have antibacterial, antimicrobial and antidepressant effects [1] [2]. However, the purification of CBG and other cannabinoids from cannabis extracts is a challenging task. This is primarily because of the complexity of the matrix, which contains terpenes, waxes, chlorophylls, and other cannabinoids. Additionally, the chemical structure and physical properties of CBG and CBD are similar, making it difficult to separate them (Fig. 1) [1] [2].
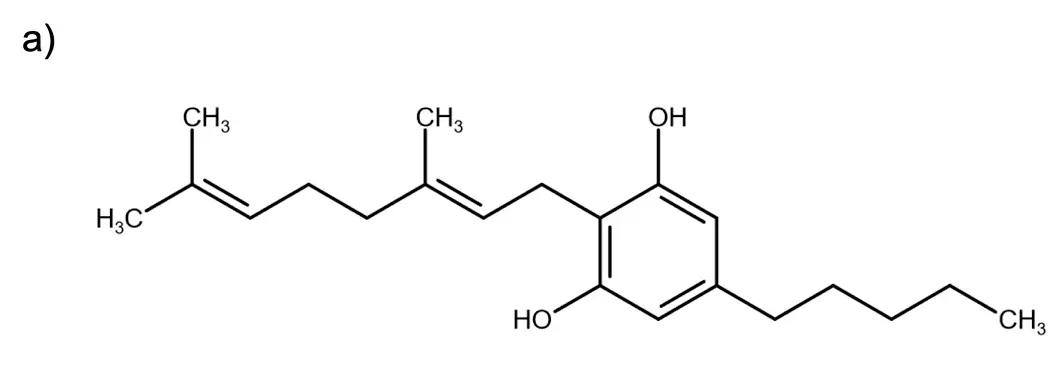
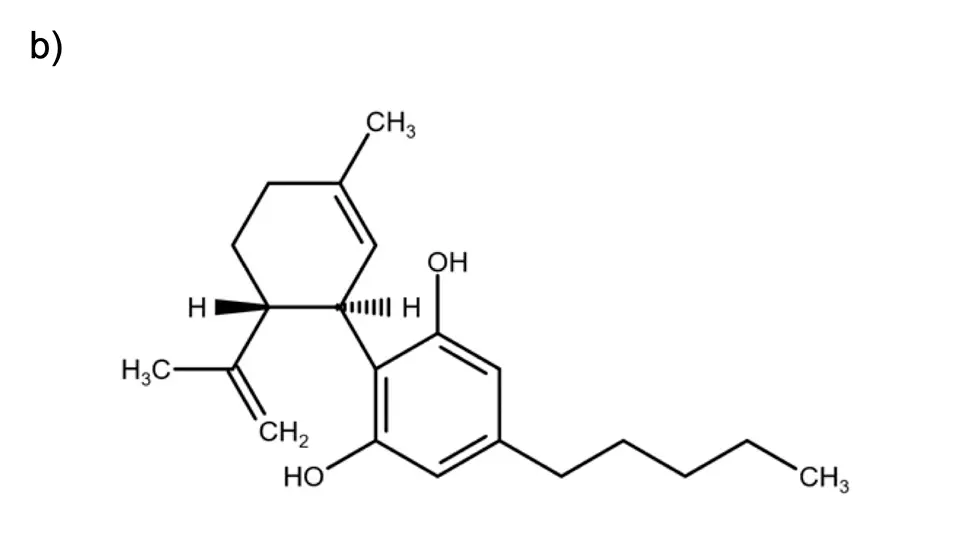
Fig. 1 Structural formula of a) CBG and b) CBD.
Therefore, specific methods and techniques are required to ensure accurate separation. Recycling chromatography is an ideal method for effectively separating two closely eluting peaks, such as CBG and CBD, by increasing the length of the column bed. The basic principle of this technique is to redirect the peaks of interest through the column multiple times, creating a simulated infinite long column, resulting in a better target peak resolution [3]. To further optimize the recycling system, two valves one for peak trapping/parking and one for recycling via the pump can be added to the system. This optimization makes it possible to remove impurities from the sample by parking the target peaks (peak parking) and reinjecting the peaks back into the system for separation over the two columns.
Sample Preparation
50 g of dried CBG-rich hemp plant material was decarboxylated at 140 °C for 30 minutes. The sample was then grinded and extracted with solvent. For this, the extraction was performed twice with ethanol (500 ml and 200 ml) by stirring for 15 minutes. Next, the extract was winterized at -20 °C for at least 24 hours to precipitate the waxes. After the winterization process, the extract was filtered with a paper filter and the solvent was removed by a rotary evaporator (43 °C water bath, 98 mbar). Finally, the extract was rapidly transferred from the evaporation flask to a container and stored in a refrigerator at 4 °C until use. For the application of this optimized recycling system, the CBG-rich cannabis extract was diluted in 100 % ethanol to the indicated concentration (i.e., 10 mg/ml).
Results
In this study, a peak recycling system with a basic set-up was upgraded to allow a peak parking step for matrix removal (step 1) before the peak recycling step (step 2). This upgrade consisted of two additional valves; a recycle valve and a peak trap valve (Fig. 2). For peak parking a column or a loop was connected to the peak trap valve.
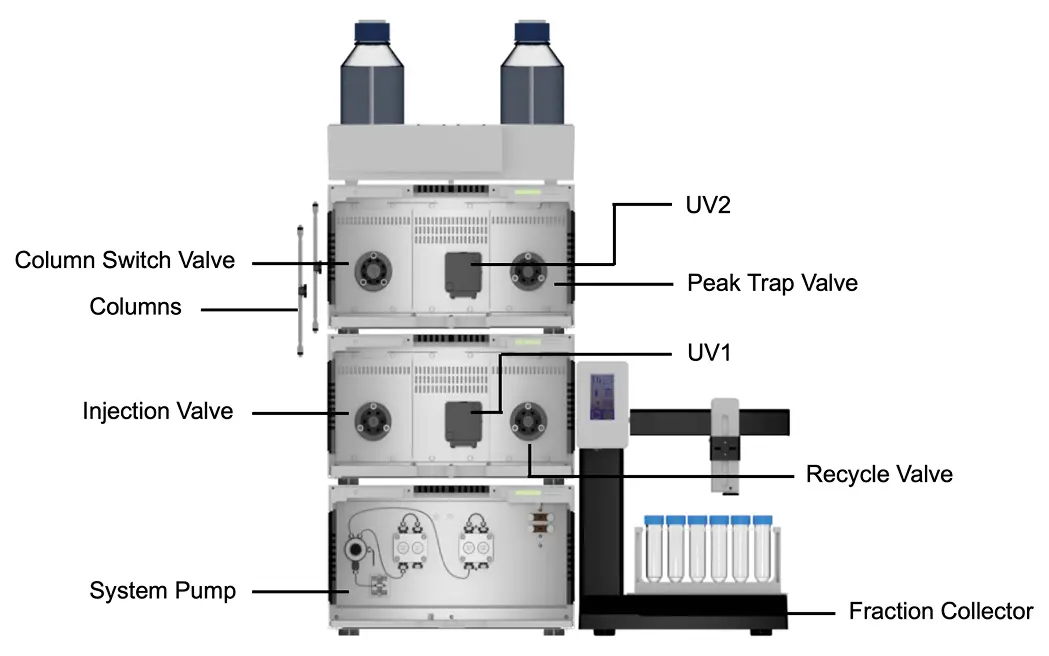
Fig. 2 Extended setup for the recycling system
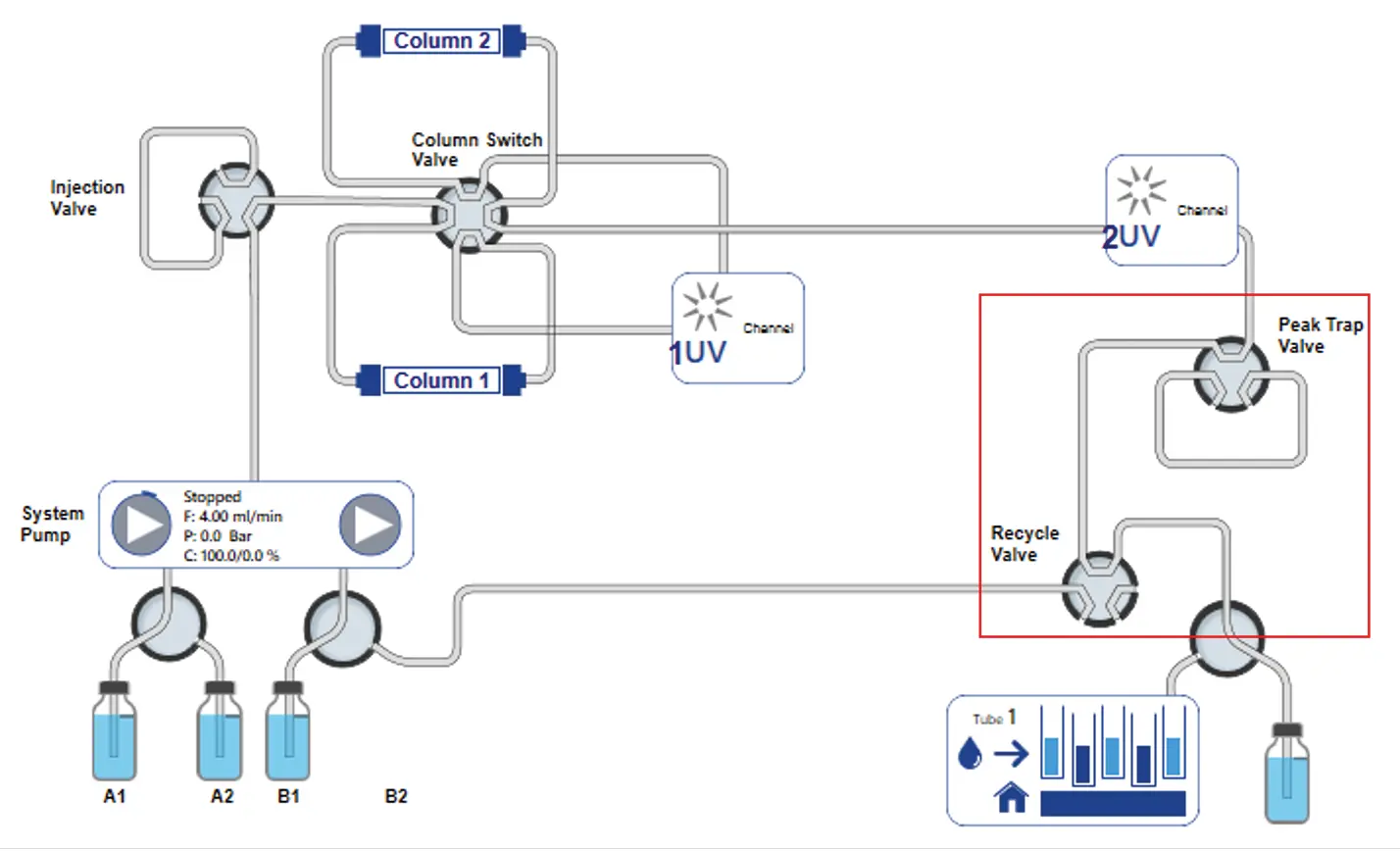
Fig. 3 PurityChrom® 6 visualization of new set-up for recycling system. Red rectangle: system upgrade.
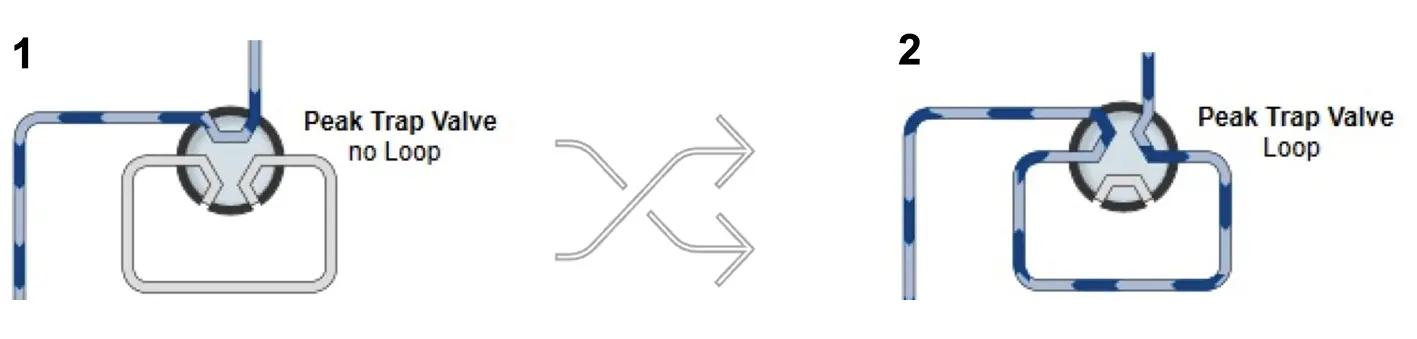
Fig. 4 Two flow paths for the peak trap valve. 1) Flow bypasses the peak trap. 2) Flow passes through the peak trap. Peak trap loop or column.
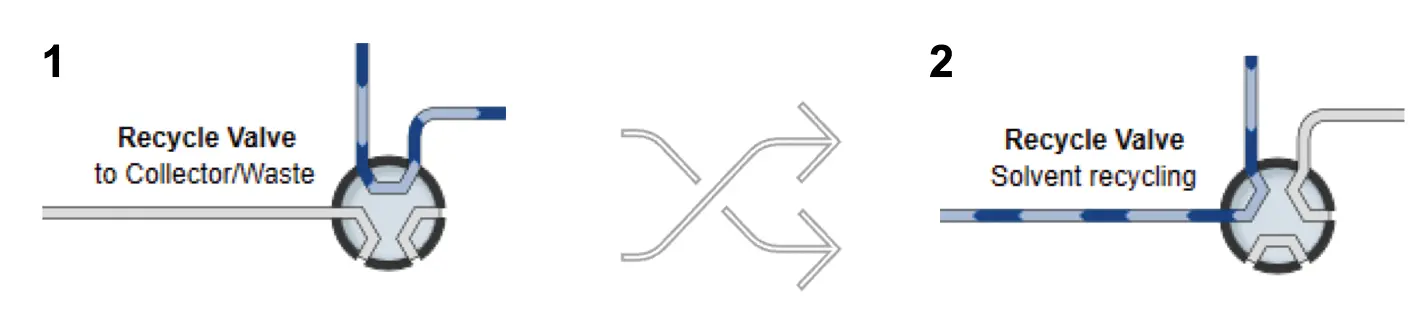
Fig. 5 Two flow paths for the recycle valve. 1) Flow goes to the collector/waste. 2) Flow is being recycled.
The visualization of the new setup of the recycling system shows an upgrade of the basic system by adding two more valves (peak trap valve and recycle valve) after the second UV detector (Fig. 3, red rectangle).
The peak trap valve can be switched between two positions, allowing the flow to bypass the trap or pass the peak into the trap (Fig. 4). By using the option to pump the sample through the peak trap, the peak can be parked in a loop or column, separating it from the sample matrix.
The recycle valve can also be switched between two positions to change the system from normal to recycle mode (Fig. 5). In addition, the eluent channel of the pump must be switched from B1 to position B2, and the eluent flow must be changed from 100 % A to 100 % B. At this point, the pump will not pump the eluent from the reservoir but recycle the solvent through the entire system. By switching the peak trap valve to position 2 the flow passes through the loop/column, reinjecting the peak back into the system and separating it over the two recycling columns to be fractionated afterwards.
Elution profile of cannabis extract
To investigate the elution behavior of the cannabis extract, ethanol based isocratic methods with different ethanol contents were tested at analytical scale. Fig. 6 shows the chromatogram of the analytical measurement using a C18 250 x 8 mm column with 10 µm particles under isocratic elution with 90:10 EtOH/H2O (v/v). The comparison between the chromatogram of the cannabis extract sample and the chromatogram of the cannabinoid CBG/CBD standards allowed their allocation. The major peak is a coelution of CBG and CBD at tR 2.60 min. A larger fraction of cannabinoids and impurities elutes in front of the major peak, a smaller amount behind. The aim of this application is: 1) to remove the early and late eluting matrix components and 2) to separate CBG from CBD by peak recycling.
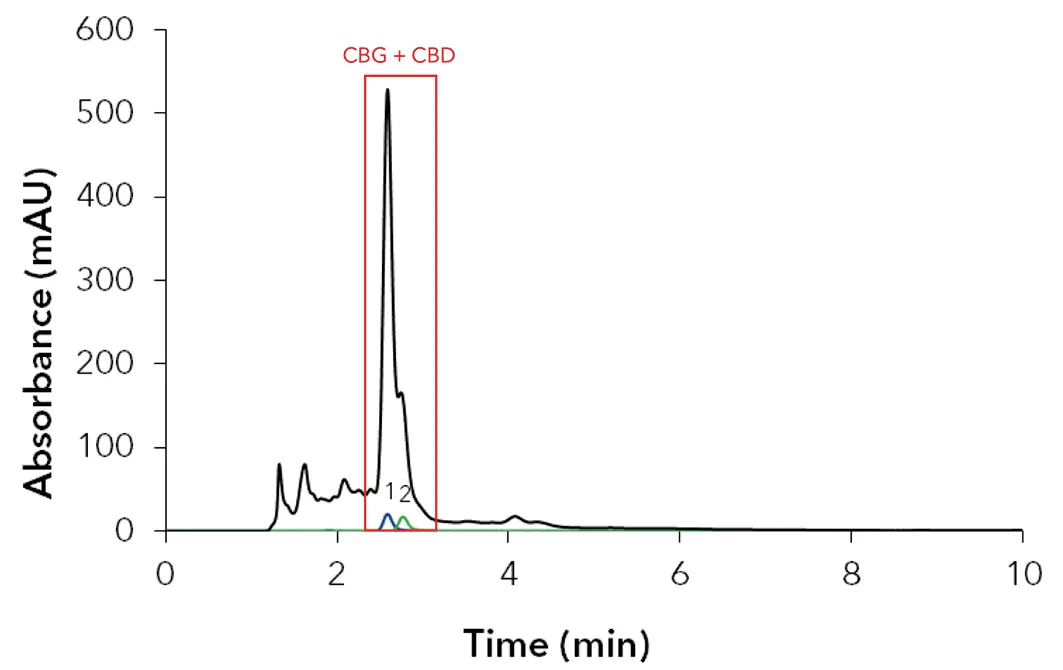
Fig. 6 Chromatogram analytical system; black: 10 mg/ml cannabis extract; blue: 25 µg/ml CBG standard; green: 25 µg/ml CBD standard; red rectangle: coelution of 1) CBG and 2) CBD; 4 ml/min; 228 nm; 10 µl injection.
Step 1: Matrix removal by Peak Parking
For the separation with the recycling system, premixed solvent was used. The column switch valve is in position 1, so that the sample first passes through column 1 and is detected by UV1, then the sample passes through column 2 and is detected by UV2. When the start of the major peak is detected by the UV2, the peak trap valve switches to position 2 and the flow passes through the peak trap where the peak of CBG/CBD is captured (Fig. 7). In the current set-up a column was used for peak parking.
As soon as the peak end is detected by the UV2, the peak trap valve is switched back to position 1, bypassing the peak trap (Fig. 8).
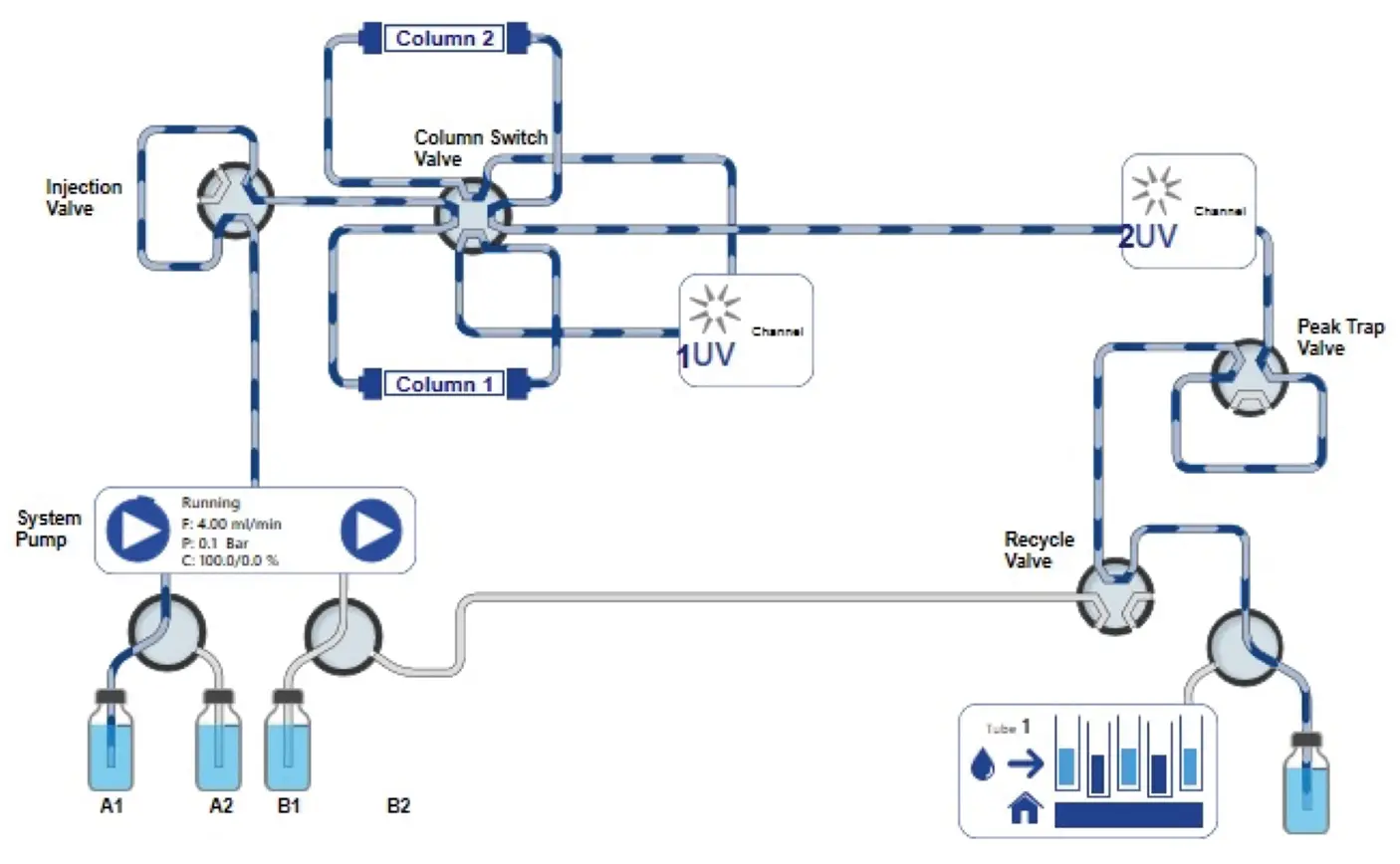
Fig. 7 PurityChrom® 6 visualization of new setup for recycling system. Flow passes the peak trap and goes to the waste.
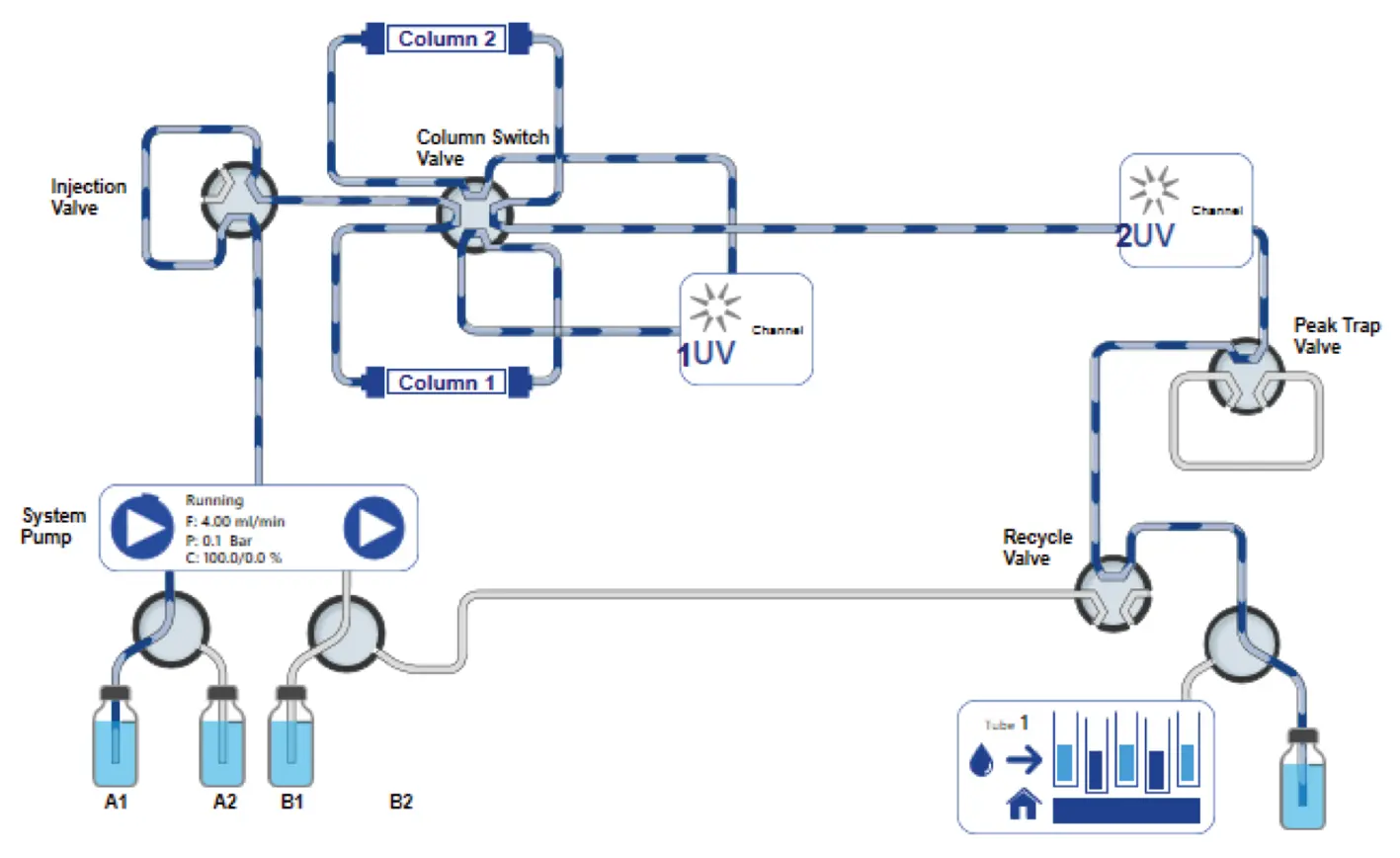
Fig. 8 PurityChrom® 6 visualization of new set-up for recycling system. Flow bypasses the peak trap and goes to the waste.
The chromatogram shown in Fig. 9 is the result after one measurement, where the peak trap valve was switched manually for peak parking to remove matrix components. The manual switching was carried out during the measurement. The valve position was switched to position 2 when the start of the major peak is detected by the UV2 and switched back in position 1 when the end of the major peak is detected by the UV2. The collection of the target peak in the trap column/loop can be automized.
Fig. 9 Chromatogram from recycling system; 1) CBG/CBD; green dash line: switching peak trap valve; blue: UV1; red: UV2; 4 ml/min; wavelength 228 nm; 100 µl injection of cannabis extract 10 mg/ml.
Step 2: Peak Recycling
After the matrix is removed the eluent channel is switched from position B1 to B2, and the eluent delivery is changed from 100 % A to 100 % B. In addition, the recycle valve and the peak trap valve are switched to position 2, reinjecting the peak to the system and recirculating the flow (Fig. 10).
When the UV1 detector detects the peak start, the eluent channel is switched back to position A1 and the eluent delivery is changed from 100 % B to 100 % A. The recycle and peak trap valve also switches back to position 1, so the flow bypasses the peak trap and goes to the waste (Fig. 11).
As the absorbance of the peak in the UV1 decreases, the position of the column switch valve is changed manually, taking the delay volume between the detector and the valve into account. Switching the column switch valve connects the second column outlet to the first column inlet. This switching cycle is repeated until the target resolution is reached, or the peak broadening exceeds one column volume. At the end, the target compounds can be collected with the fraction collector. The automated fractionation was carried out by overstep the threshold set for the UV2 in 15 seconds intervals with 1 ml fraction volume.
The chromatogram in Fig. 12 shows the result with the fractionation parameters for 13 collected fractions.
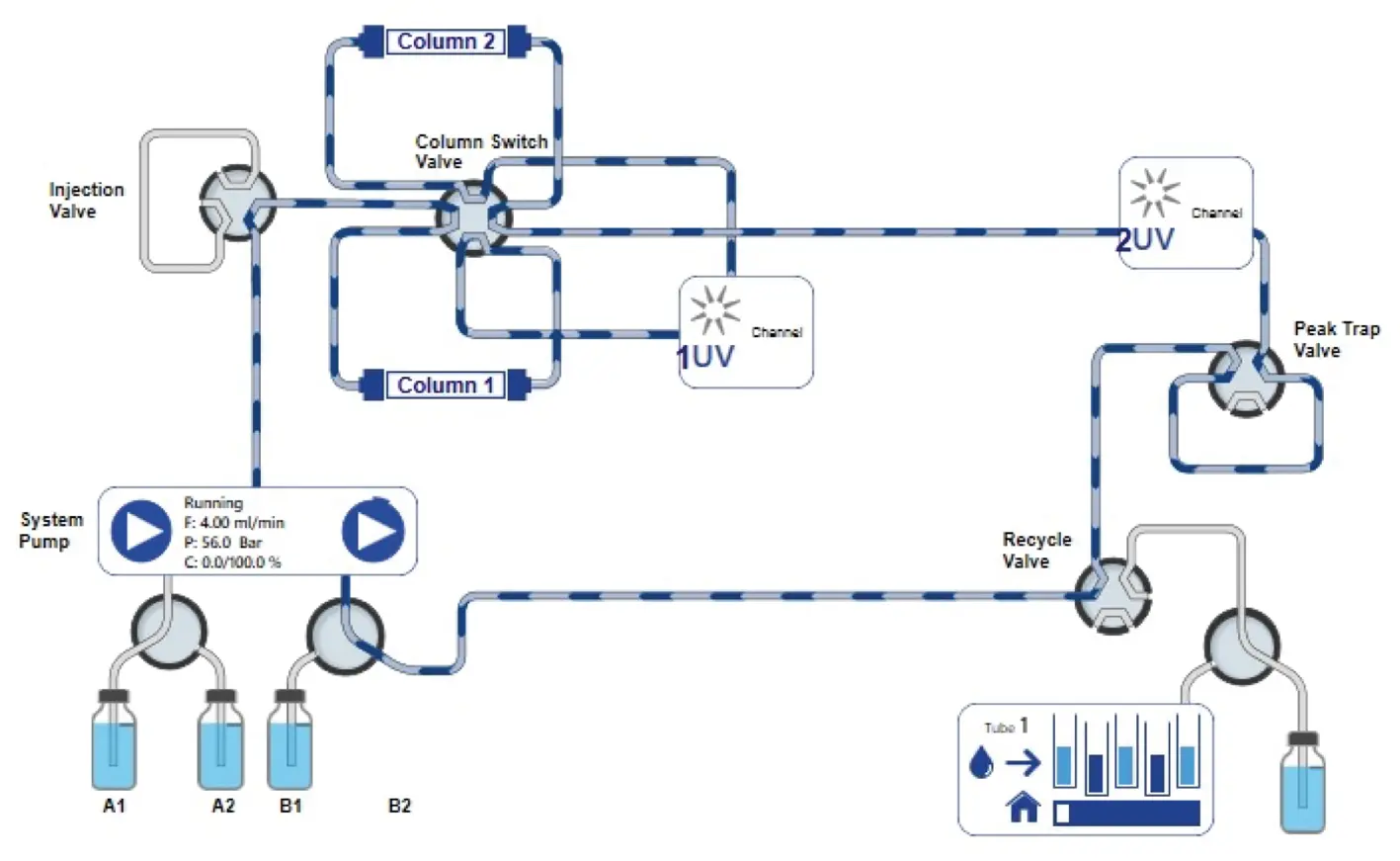
Fig. 10 PurityChrom® 6 visualization of new setup for recycling system. Flow goes through the peak trap and becomes recycled.
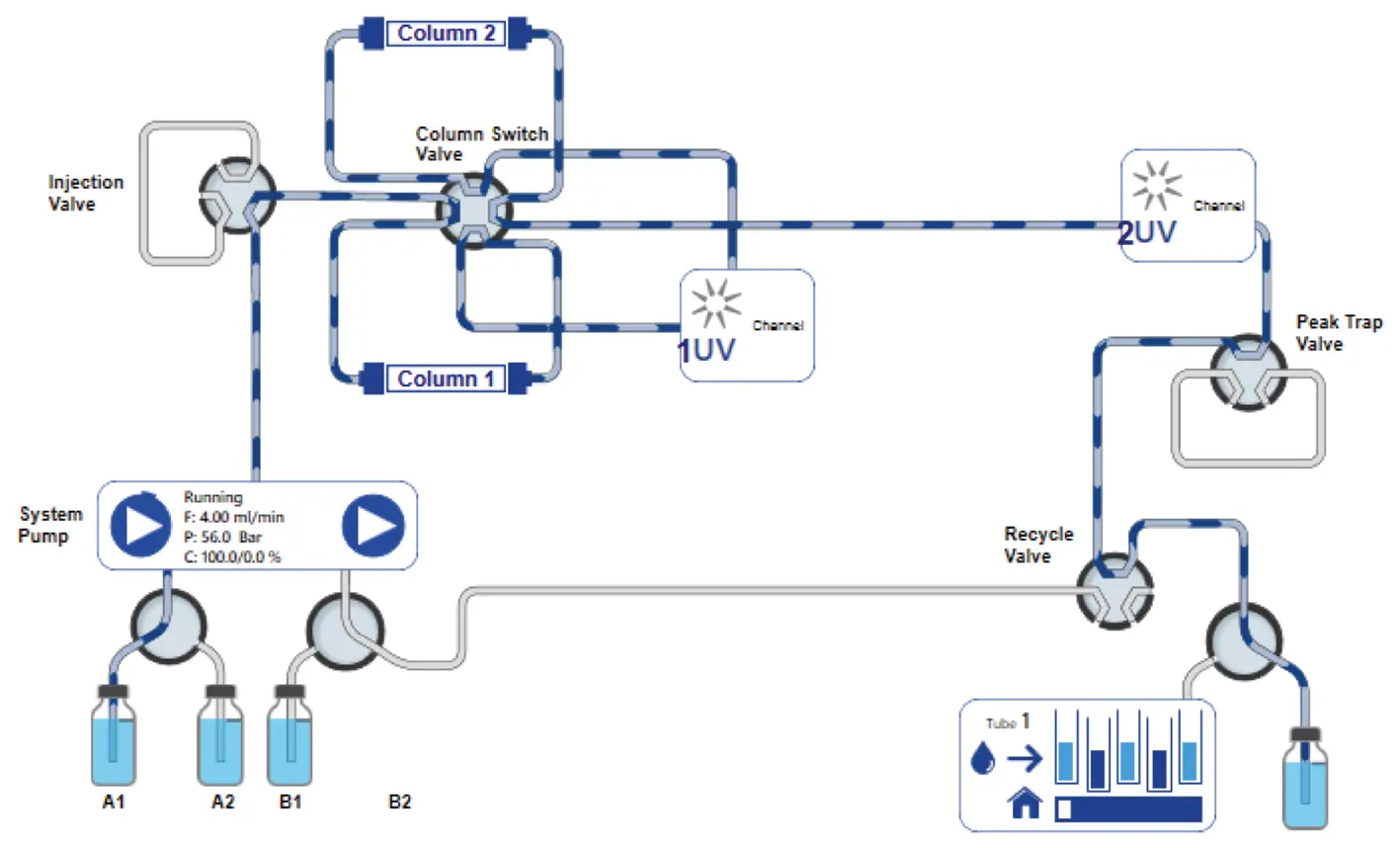
Fig. 11 PurityChrom® 6 visualization of new set-up for recycling system. Flow bypasses the peak trap and goes to the waste.
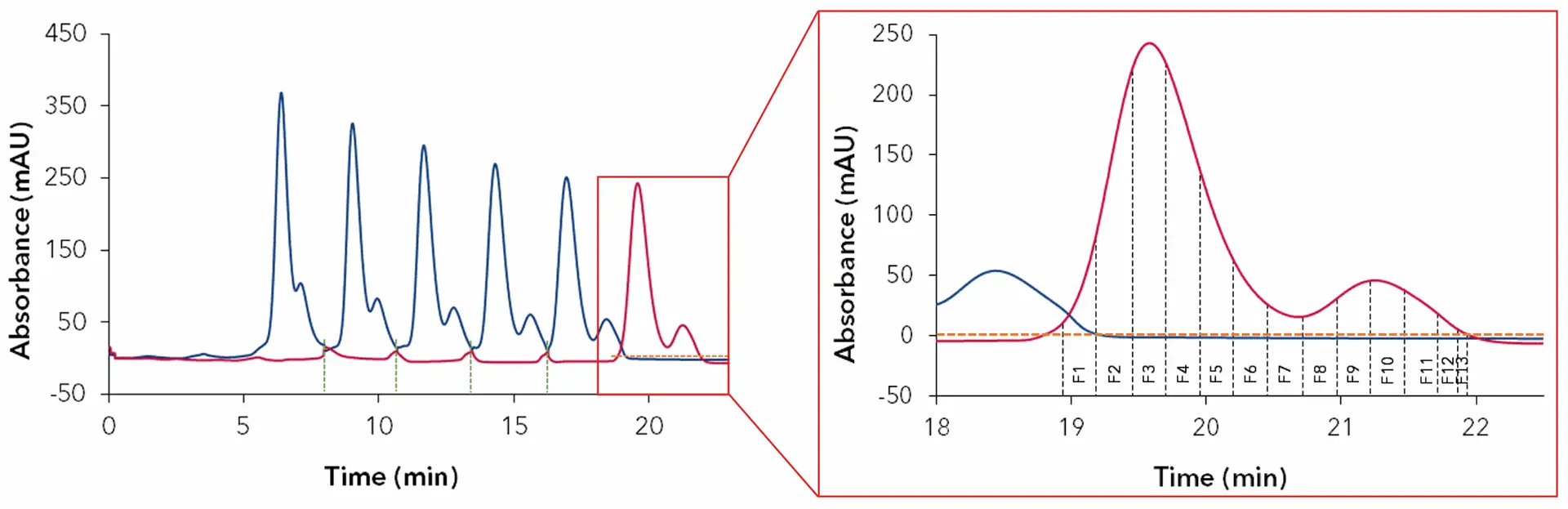
Fig. 12 Chromatogram from recycling system; green dash line: switching peak trap valve; blue: UV1; red: UV2; orange dash line: threshold 5 mAU for the UV2; 4 ml/min; wavelength 228 nm; 100 µl injection of cannabis extract 10 mg/ml.
Fractions 1 to 13 were analyzed by the analytical method [4] to calculate the amount of CBG and CBD and to evaluate the separation between them. As in the previous measurement, none of the early and late eluting matrix components were found in the fractions (Fig. 13).
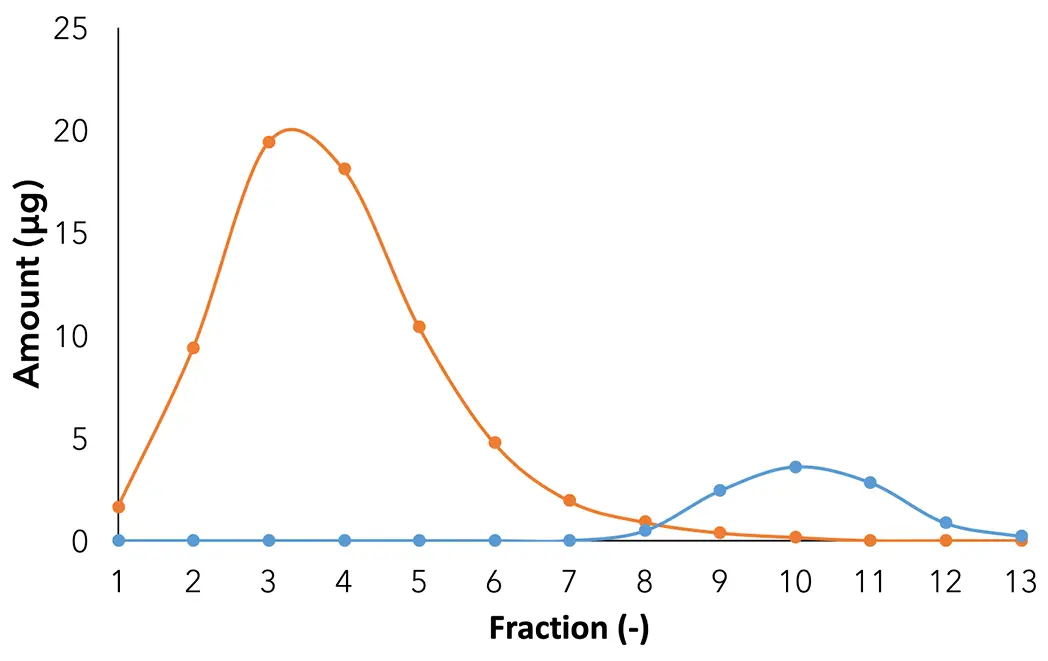
Fig. 13 Amount profile of CBG and CBD in each fraction: orange: CBG; blue: CBD.
The amount and purity of the identified cannabinoids were determined for the sample (10 mg/ml cannabis extract) and the collected fractions (Tab. 1).
Tab. 1 Analysis of fractions from preparative purification (100 µl injection with 10 mg/ml cannabis extract). For each fraction the amount and purity of identified cannabinoids are shown.
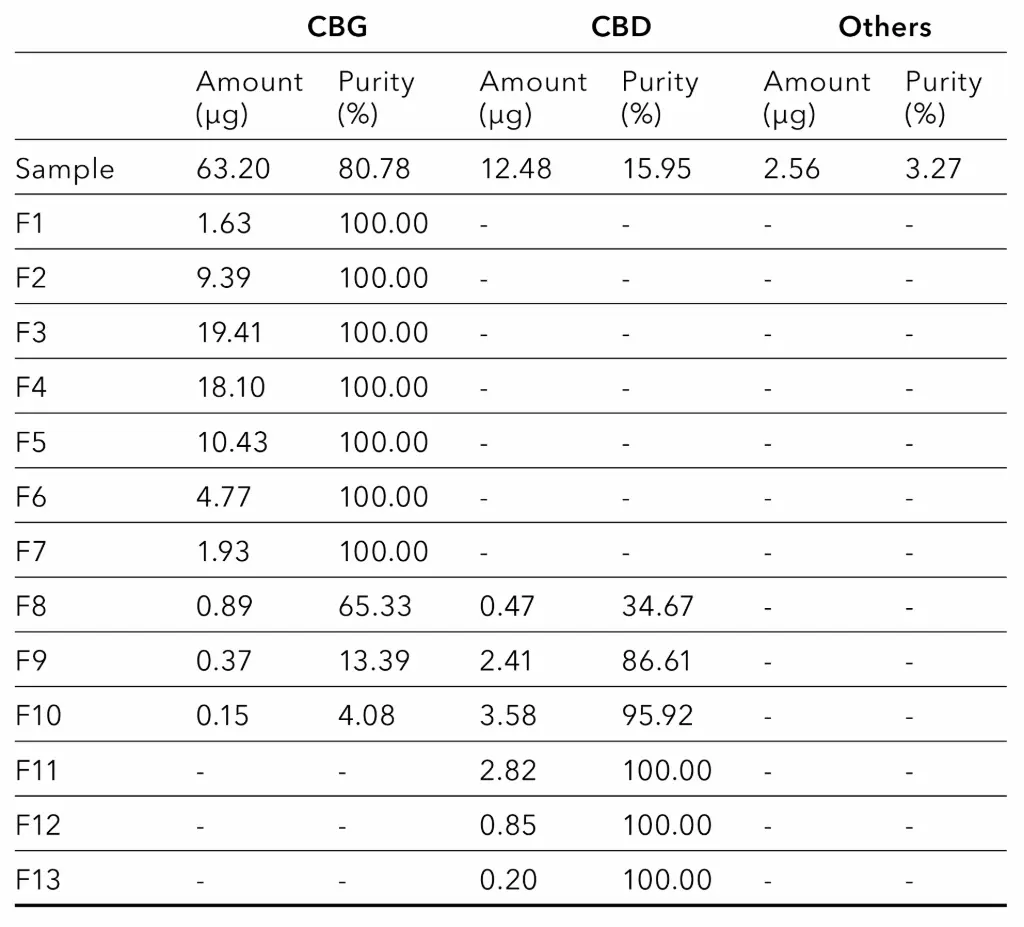
The analysis shows that fractions 1 to 7 contain only CBG, with no other detectable impurities. Therefore, the purity of CBG in these fractions is 100 %. After fraction 7, the purity of CBG decreases in each subsequent collected fraction due to the presence of CBD. In contrast, the purity of CBD increases. In fractions 11 to 13 only CBD is present, so the purity of CBD in these fractions is 100 %.
Based on the amount and purity of CBG and CBD in each collected fraction, theoretical values can be determined for both cannabinoids in selected fraction groups (Tab. 2 and 3). This allows the selection of fractionation windows in which CBG and CBD can be fractionated with the highest possible recovery or purity.
Tab. 2 The theoretically amount, purity and loss of CBG in selected fraction groups.
Amount (µg) | Purity (%) | Loss (%) | |
F1 to F7 | 65.65 | 100.00 | 2.11 |
F1 to F8 | 66.54 | 99.30 | 0.78 |
F1 to F9 | 66.91 | 95.87 | 0.23 |
F1 to F10 | 67.07 | 91.21 | 0.00 |
Tab. 3 The theoretically amount, purity and loss of CBD in selected fraction groups.
Amount (µg) | Purity (%) | Loss (%) | |
F11 to F13 | 3.86 | 100.00 | 62.62 |
F10 to F13 | 7.44 | 97.99 | 27.94 |
F9 to F13 | 9.86 | 94.94 | 4.57 |
F8 to F13 | 10.33 | 87.95 | 0.00 |
The presented Tab. 2 and 3 show that a completely pure CBG or CBD fraction is associated with a loss of the respective cannabinoid. For example, if fractions 1 to 7 are pooled, the purity of CBG would be 100 %, but a small amount of CBG will be lost. In contrast, if more fractions are pooled, such as fractions 1 to 10, the highest possible recovery would be obtained, but with a lower purity for CBG. In this case, a purity of about 91 % could be expected for CBG. The same is valid for CBD. For example, if fractions 11 to 13 are combined, the purity of CBD would be 100 %, but over 60 % of CBG would be lost. If, in contrast, more fractions are combined, such as fractions 8 to 13, the purity would decrease from 100 % to about 88 %, but the loss would be reduced from 63 % to 0 %. The higher measured amount of CBG (Tab. 2) compared to the sample (Tab. 1) is due to recovery rates of > 100 %.
Conclusion
An optimized recycling system was used to remove matrix components from CBG-rich cannabis extracts, which enabled the subsequent separation of CBG and CBD. For this purpose, a peak trap valve and a recycle valve were added to the system after the UV2. The peak trap valve was used to remove the early and late eluting matrix components. The recycle valve was used for peak recycling via the pump. The analysis of the cannabis extract showed that the main cannabinoid was CBG with a purity of 81 %. A preparative ethanol based isocratic method was developed to purify CBG from cannabis extract with high purity and yield. From a 100 µl sample injection (10 mg/ml cannabis extract), approximately 66 µg CBG was purified with 100 % purity. In addition, the purity of CBD could be increased between 88 % and 100 % depending on the fractionation window. The results demonstrate the simplicity and benefits of optimizing the recycling system. The shown method is an example and was targeted at two cannabinoids. Depending on the target, the method can be adapted to other target compounds from different matrices.
Material and Methods
Tab. 4 Instrument: Recycling system configuration (used set-up)
Instrument | Description | Article No. |
Pump | AZURA P 6.1L, HPG 50 ml pump ceramic head | |
Assistant 1 | AZURA Assistant ASM 2.2.L Left module: valve drive VU 4.1 Middle module: UVD 2.1S Right module: valve drive VU4.1 | |
Recycle Valve | 2-position valve, 6 ports, sst, 1/16" | |
Injection Valve | 2-position valve, 6 ports, sst, 1/16" | |
Assistant 2 | AZURA Assistant ASM 2.2.L Left module: valve drive VU4.1 Middle module: UVD 2.1S Right module: valve drive VU4.1 | |
Column switch Valve | 2-position valve, 8 ports, sst, 1/16" | |
Peak Trap Valve | 2-position valve, 6 ports, sst, 1/16" | |
Flow cell (UVD1) | Semi-preparative 3 mm UV Flow Cell 3 mm path length, 1/16", 2 µl volume, 300 bar, sst | |
Flow cell (UVD2) | Semi-preparative 3 mm UV Flow Cell 3 mm path length, 1/16", 2 µl volume, 300 bar, sst | |
Column for Peak Parking | Eurospher II C18, 5 µm; 100 Å, | |
Columns for Peak Recycling | 2 x Eurospher II C18, 10 µm, | |
Capillaries | PEEK 1/16", 0.5 mm | |
Fraction Collector | For 1/16" or 1/8" tubing | |
Software | PurityChrom® 6 full license |
The analytical measurements were performed by using the cannabis profiler HPLC system for quality control of cannabis according to the German Pharmacopoeia (SYS986402200).
Tab. 5 Method: Recycling system
Parameter | Wert |
Flowrate | 4 ml/min |
Data rate | 2 Hz |
Temperature | Ambient |
Injection volume | 100 µl |
Wavelengths | 228 nm |
Mob. Phase | EtOH/H2O (90:10) |
Isocratic | EtOH/H2O (90:10) |
References
[1] De Luca, C., Krauke, Y., Stephan, S., Greco, G., Compagnin, G., Buratti, A., Cavazzini, A., Catani, M. & Felletti, S. Green cannabigerol purification through simulated moving bed chromatography. Green Analytical Chemistry, 100066 (2023).
[2] Nachnani, R., Raup-Konsavage, W. M., Vrana, K. E. The pharmacological case for cannabigerol. Journal of Pharmacology and Experimental Therapeutics, 376(2), 204-212 (2020).
[3] G. Compagnin, Y. Krauke, S. Felletti, G. Greco. Improved recycling chromatography – how to make faster and automatic separations of peak pairs. KNAUER Wissenschaftliche Geräte GmbH (2023) https://www.knauer.net/en/improved-recycling-chromatography-how-to-make-faster-and-automatic-separations-of-peak-pairs/a46707
[4] Loxterkamp, L., Monks, K. (C)an(n)alyze: determination of 16 cannabinoids inside flowers, oils and seeds. KNAUER Wissenschaftliche Geräte GmbH (2020) https://www.knauer.net/en/cannalyze-determination-of-16-cannabinoids-inside-flowers-oils-and-seeds/a34489
Application details
Method | Preparative HPLC |
Mode | RP |
Substances | Cannabigerol (CBG), Cannabidiol (CBD) |
CAS number | 25654-31-3, 13956-29-1 |
Version | Application No.: VPH0077 Version 1 / 11/2023 | ©KNAUER Wissenschaftliche Geräte GmbH |

